When an aqueous solution of LiCl is electrolyzed using graphite electrodes, as the current flows, the pH of the solution around cathode ……………………
Video Solution
Text Solution
The correct Answer is:(Increaese)
( Increases )
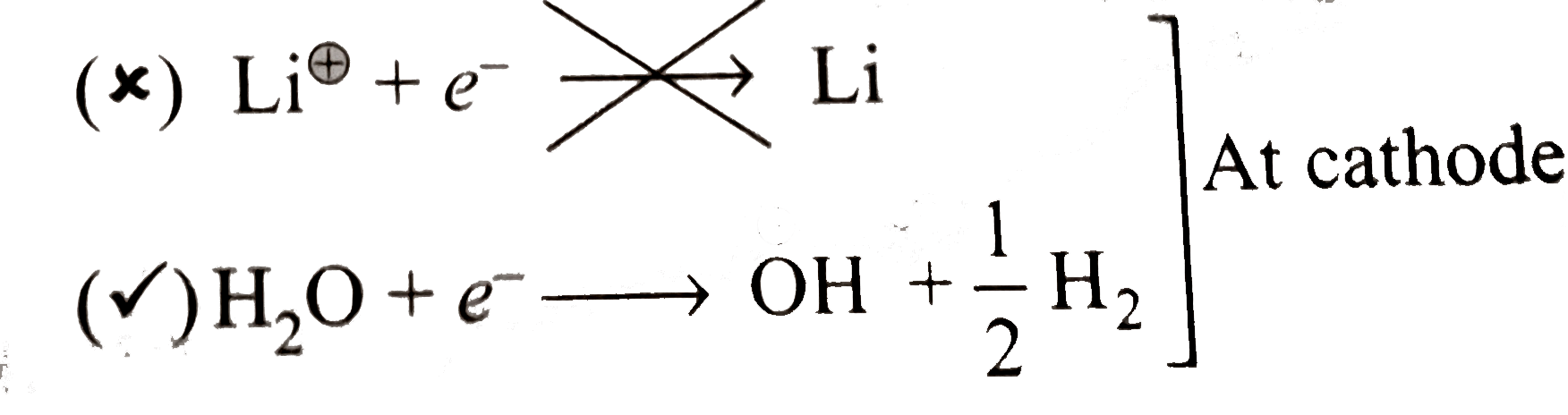
Due to the formation of c−OH ions at cathode, pOH decreases and pH increases.
|
Similar Questions
Knowledge Check
When an aqueous solution of lithium chloride is electrolysed using graphite electrodes
ACl2 is liberated at the anode.BLi is deposited at the cathodeCas the current flows,pH of the solution around the cathode remains constantDas the current flows,pH of the solution around the cathode decreasesWhen an aqueous solution of CaCl2 is electrolyzed using inert electrods, which of the following is ( are ) true ?
ACalcium deposites on cathode.BCalcium deposits an anodeCChloride is liberated on anodeDCalcium hydroxide precipitates near cathode on prolonged hydrolysisAn aqueous solution with pH=0 is
AacidicBalkalineCneutralDamphoteric .
The aqueous solution whose pH =0 is-
View SolutionWhen an aqueous concentrated solution of lithium chloride is electrolysed using inert electrodes:
View SolutionWhen an aqueous solution of sodium choride is electrolysed using platinum electrodes, the compounds discharged at the electrodes are
View SolutionThe aqueous solution whose pH =0 is-
View SolutionAqueous NaCl solution is electrolyzed using platinum electodes. What is the product formed at cathode?
View Solution
