The following data were obtained during the first thermal decompoistion of N2O5(g) at constant volume.
2N2O5(g)→2N2O4(g)+O2(g)
∣∣
∣
∣∣S.No.Time (s)Total pressure (atm)i.00.5ii.1000.512∣∣
∣
∣∣
Calculate the rate constant.
To calculate the rate constant for the thermal decomposition of N2O5, we will follow these steps:
Step 1: Write the balanced chemical equation
The balanced equation for the decomposition of N2O5 is:
2N2O5(g)→2N2O4(g)+O2(g)
Step 2: Determine the initial and final pressures
From the data provided:
- At time t=0 s, the total pressure P0=0.5 atm.
- At time t=100 s, the total pressure Pt=0.512 atm.
Step 3: Relate the change in pressure to the change in concentration
Let x be the change in pressure due to the decomposition of N2O5. According to the stoichiometry of the reaction:
- For every 2 moles of N2O5 that decompose, 2 moles of N2O4 and 1 mole of O2 are produced.
- Therefore, the change in pressure can be expressed as:
Pt=P0−2x+x=P0−x
This is because 2 moles of N2O5 produce 1 mole of O2 and 2 moles of N2O4.
Step 4: Calculate the change in pressure
From the given data:
Pt=0.512 atm
P0=0.5 atm
Substituting into the equation:
0.512=0.5−x
Thus,
x=0.5−0.512=−0.012 atm
Step 5: Calculate the change in pressure due to N2O5
Since x represents the decrease in pressure due to the decomposition of N2O5:
P0−Pt=0.5−0.512=−0.012 atm
This means that the pressure increase is due to the formation of products.
Step 6: Calculate the rate constant k
For a first-order reaction, the rate constant k can be calculated using the following formula:
k=2.303tlog(P0Pt)
Where:
- t=100 s
- P0=0.5 atm
- Pt=0.512 atm
Substituting the values:
k=2.303100log(0.50.512)
Step 7: Calculate the logarithm
First, calculate the ratio:
0.50.512≈0.9765625
Now, calculate the logarithm:
log(0.9765625)≈−0.0104
Step 8: Final calculation of k
Now substitute back into the equation for k:
k=2.303100×(−0.0104)
k≈−0.000239 atm−1s−1
Step 9: Convert to appropriate units
Since we want the rate constant in standard units, we can express it as:
k≈2.39×10−4 s−1
Final Answer
The rate constant k for the decomposition of N2O5 is approximately:
k≈2.39×10−4 s−1
---
The balanced equation for the decomposition of N2O5 is:
2N2O5(g)→2N2O4(g)+O2(g)
From the data provided:
- At time t=0 s, the total pressure P0=0.5 atm.
- At time t=100 s, the total pressure Pt=0.512 atm.
Let x be the change in pressure due to the decomposition of N2O5. According to the stoichiometry of the reaction:
- For every 2 moles of N2O5 that decompose, 2 moles of N2O4 and 1 mole of O2 are produced.
- Therefore, the change in pressure can be expressed as:
Pt=P0−2x+x=P0−x
This is because 2 moles of N2O5 produce 1 mole of O2 and 2 moles of N2O4.
From the given data:
Pt=0.512 atm
P0=0.5 atm
0.512=0.5−x
Thus,
x=0.5−0.512=−0.012 atm
Since x represents the decrease in pressure due to the decomposition of N2O5:
P0−Pt=0.5−0.512=−0.012 atm
This means that the pressure increase is due to the formation of products.
For a first-order reaction, the rate constant k can be calculated using the following formula:
k=2.303tlog(P0Pt)
Where:
- t=100 s
- P0=0.5 atm
- Pt=0.512 atm
k=2.303100log(0.50.512)
First, calculate the ratio:
0.50.512≈0.9765625
Now, calculate the logarithm:
log(0.9765625)≈−0.0104
Now substitute back into the equation for k:
k=2.303100×(−0.0104)
k≈−0.000239 atm−1s−1
Since we want the rate constant in standard units, we can express it as:
k≈2.39×10−4 s−1
The rate constant k for the decomposition of N2O5 is approximately:
k≈2.39×10−4 s−1
Similar Questions
The following data were obtained during the first order thermal decomposition of N2O5 (g) at constant volume:
2N2O5(g)→2N2O4(g)+O2(g)
(S.no,Time,Total Pressure / atm),(1,0,0,5),(2,100,0.512)
Calculate the rate constant.
View SolutionThe following data were obtained the first order thermal decomposition of N2O5(g) at constant volume : 2N2O5(g)→2N2O4(g)+O2(g)
S. No.Time/sTotal pressure (atm)100.521000.512
Calculate the rate constant.
View Solution
Knowledge Check
The following data were obtained during the first order thermal
decomposition of SO2Cl2 at a constant volume SO2Cl2(g)→SO2(g)+Cl2(g)
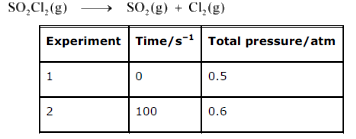
Calculate the rate of the reaction when total pressure is 0.65 atmA0.35atmS−1B2.235×10−3atmS−1C7.8×10−4atms−1D1.55×10−4atmS−1For the first order reaction
2N2O5(g)→4NO2(g)+O2(g)AThe concentration of the reactant decreases exponentially with time.BThe half life of the reaction decreases with increaisng temperature.CThe half life of the reaction depends on the initial concentration of the reactant.DThe reaction proceeds to 99.6% completion in 8 half. Life durations.For the first order reaction
2N2O5(g)→4NO2(g)+O2(g)AThe concentration of the reaction decreases exponentially with timeBThe half-life of the reaction decreases with increasing temperatureCThe half-life of the reaction depends on the initial concentration of the reactantDThe reaction proceeds to 99.6 % completion in eight half-life duration
The following data were obtained during the first order thermal decomposition of N2O5(g) at constant volume.
2N2O5(g)→2N2O2(g)+O2(g)

Calculate the rate constant for the gaseous reaction.
View SolutionThe following data were obtained during the first order thermal decomposition of N2O5 at constant volume :
2N2O5→2N2O4+O2

Calculate rate constant.
View SolutionThe following data were obtained during the first order thermal
decomposition of SO2Cl2 at a constant volume SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate of the reaction when total pressure is 0.65 atm
View SolutionThe following rate data were obtained for the thermal decomposition of N2O5(g)
2N2O5(g)→2N2O4(g)+O2(g)
Time (sec)050Total pressure (atm)0.20.25
Calculate the reaction rate when the total pressure is 0.28 atm
View SolutionFor the first order reaction
2N2O5(g)→4NO2(g)+O2(g)
View Solution
