0.246 gm of an organic compound containing 58.53% carbon and 4.06% hydrogen gave 22.4 ml of nitrogen at STP. What is the empirical formula of the compound?
Video Solution
Text Solution
calculation of composition:
(i). Percentage of carbon =58.53 (given)
(ii). Percentage of hydrogen =4.06 (given)
(iii) percentage of nitrogen
=28×vol.ofN2 at STP×10022400×Wt. Of compound
=28×22.4×10022400×0.246=11.37%
(iv) percentage of oxygen =100− ("percentage of "C+" percentage of" H+" percentage of N")
=100−(58.53+4.06+11.38)=26.03
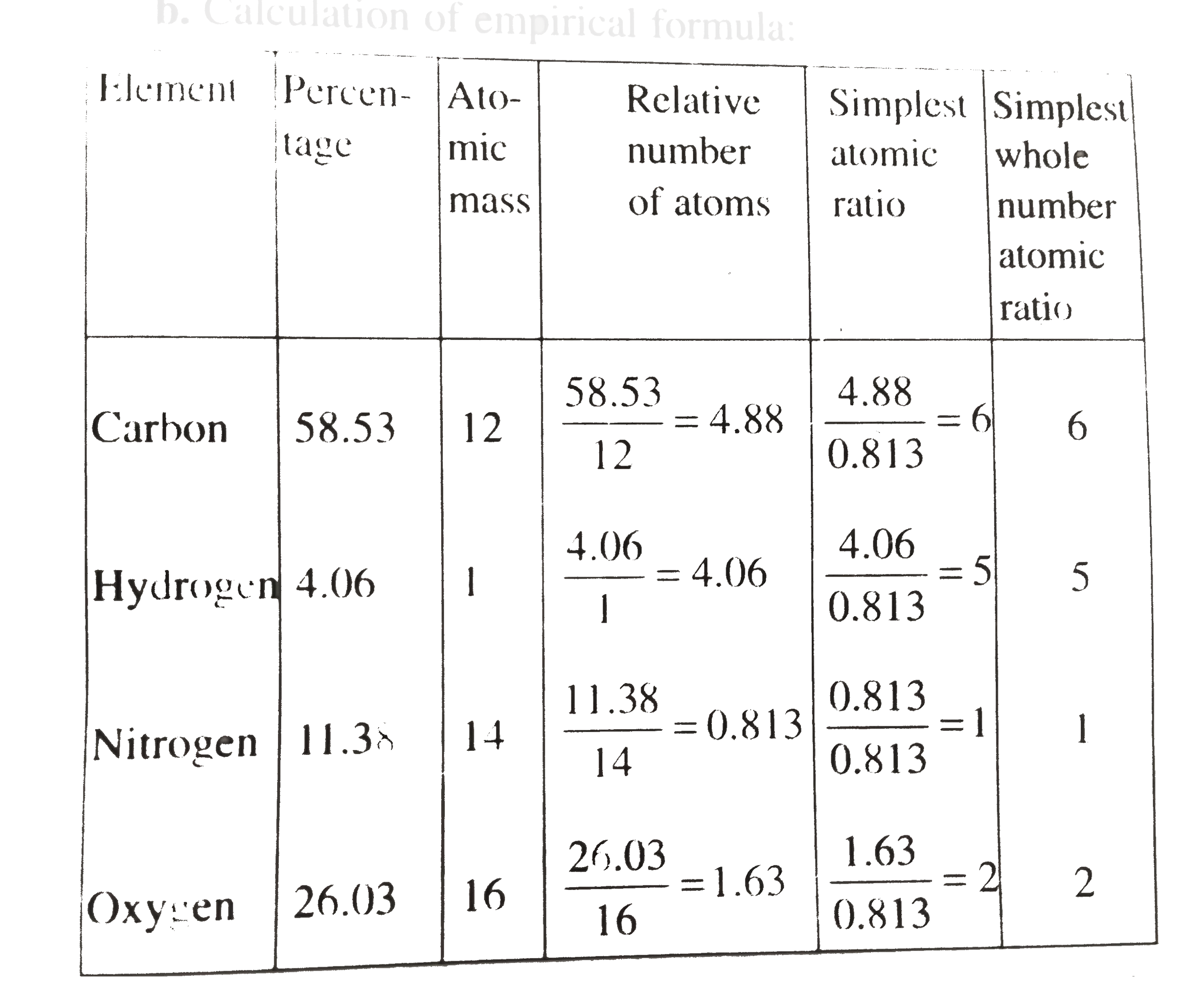
Hence, the empirical formula of the compound is C6H5NO2.
|
Similar Questions
Knowledge Check
0.246 g of an organic compound containing 58.53% carbon and 4.06% hydrogen gave 22.4 mL of nitrogen at STP. What is the empirical formula of the compound?
AC2H7N2BC6H5NO2CC2H7NO2DC2H2NO20.365 g of an orgainc compound containing nitrogen gave 56 ml nitrogen at S.T.P. The percentage nitrogen in the given compound is
A19.18B38.36C9.18D29.18An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71% and H, 9.67%. The empirical formula of the compound would be
ACHOBCH4OCCH3ODCH2O
A compound contains 34.8% Oxygen, 52.2% carbon and 13.0% Hydrogen. What is the empirical formula of the compound ?
View SolutionA compound contains 34.8% oxygen, 52.2% carbon and 13.0% hydrogen. What is the empirical formula mass of the compound?
View SolutionAn organic compound contained 78%(by wt.) carbon and remaining % of hydrogen. The right option for the empirical formula of this compound is:
View SolutionAn organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gaveC,38.71% and H,9.67%. The empirical formula of the compound would be :
View SolutionAn organic compound contains carbon, hydrogen and oxygen. Its chemical analysis gave C, 38.71% and H, 9.67%. The empirical formula of compound would be
View Solution

