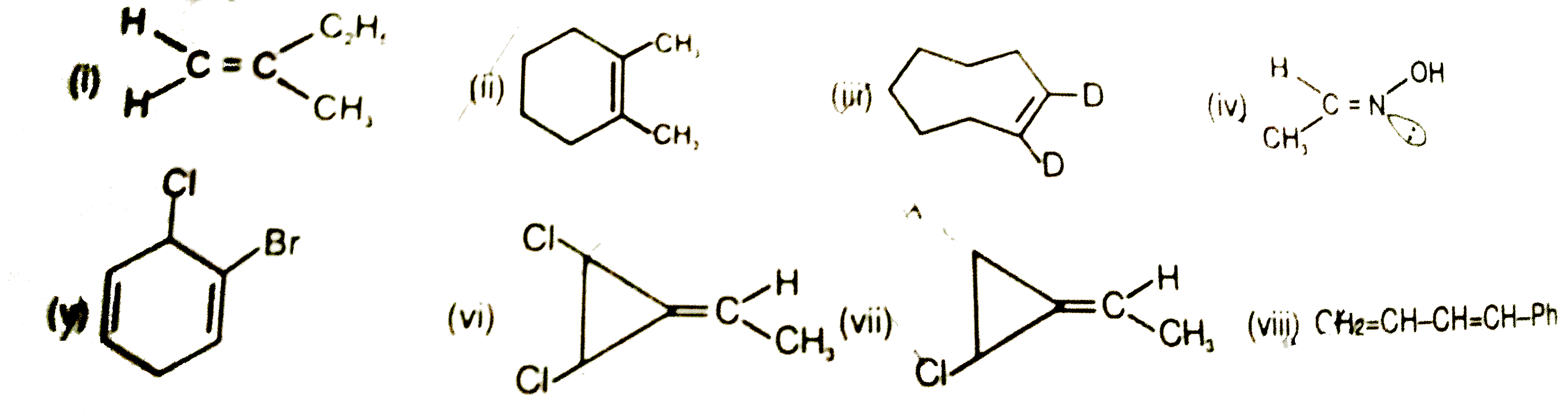
Which of the following will show geometrical isomerism ?
A
CH3−C2H5∣C=CH2
B
[Pt(NH3)2Cl2]
C
[Cr(NH3)4Cl2]⊕
D

Video Solution
Text Solution
The correct Answer is:B, C, D
a. Does not show G.I. Due to two same group (H) on the double bonds.
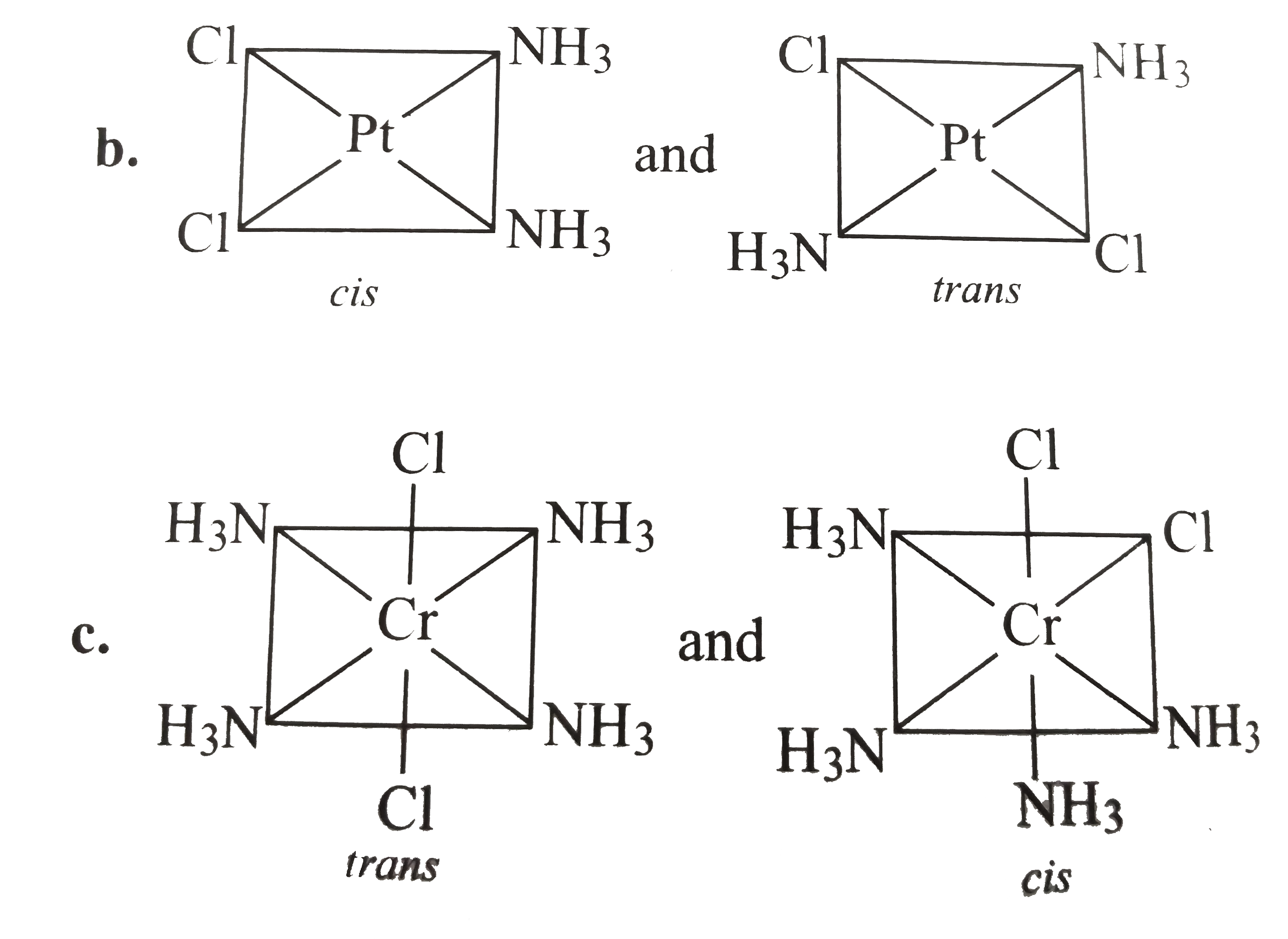
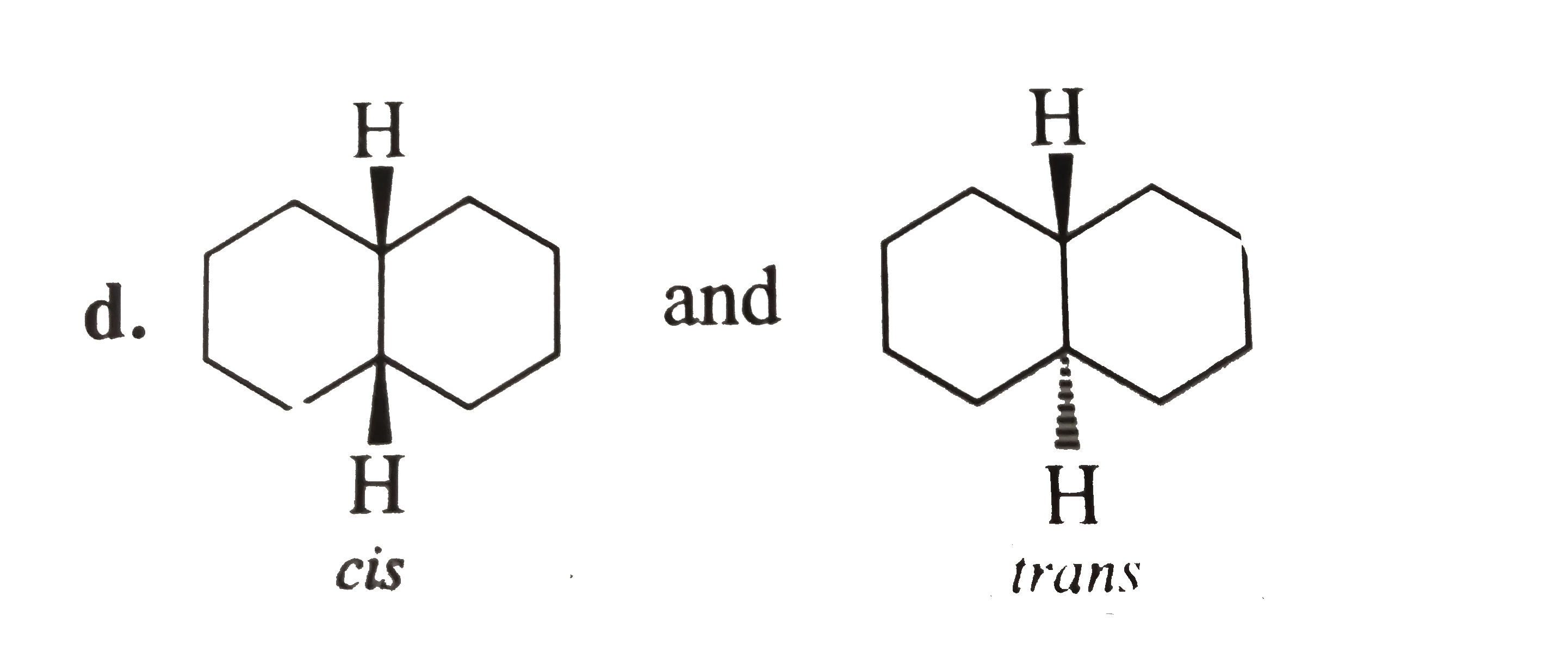
|
Similar Questions
Knowledge Check
Which of the following can show geometrical isomerism?
A⎡⎢ ⎢⎣Pt⎛⎜ ⎜⎝NH2−C∣CH3H−C∣CH3H−NH2⎞⎟ ⎟⎠2⎤⎥ ⎥⎦2+B[Co(en)2NH3Cl]2+CBoth (a) and (b)Dnone of theseWhich of the following can show geometrical isomerism?
A⎡⎢ ⎢⎣Pt⎛⎜ ⎜⎝NH2−C∣CH3H−C∣CH3H−NH2⎞⎟ ⎟⎠2⎤⎥ ⎥⎦2+B[Co(en)2NH3Cl]2+CBoth (a) and (b)Dnone of theseWhich of the following can show geometrical isomerism?
A⎡⎢ ⎢⎣Pt⎛⎜ ⎜⎝NH2−CH∣CH3−CH∣CH3−NH2⎞⎟ ⎟⎠2⎤⎥ ⎥⎦2+B[Co(en)2(NH3)Cl]2+CBoth a and BDNone if these
Which of the following will not show geometrical isomerism ?
View SolutionWhich of the following will not show geometrical isomerism?
View SolutionWhich of the following will not show geometrical isomerism?
View SolutionWhich of the following will not show geometrical isomerism?
View SolutionWhich of the following will not show geometrical isomerism?
View Solution