Find the number of atoms per unit cell in the following crystal structures :
Text Solution
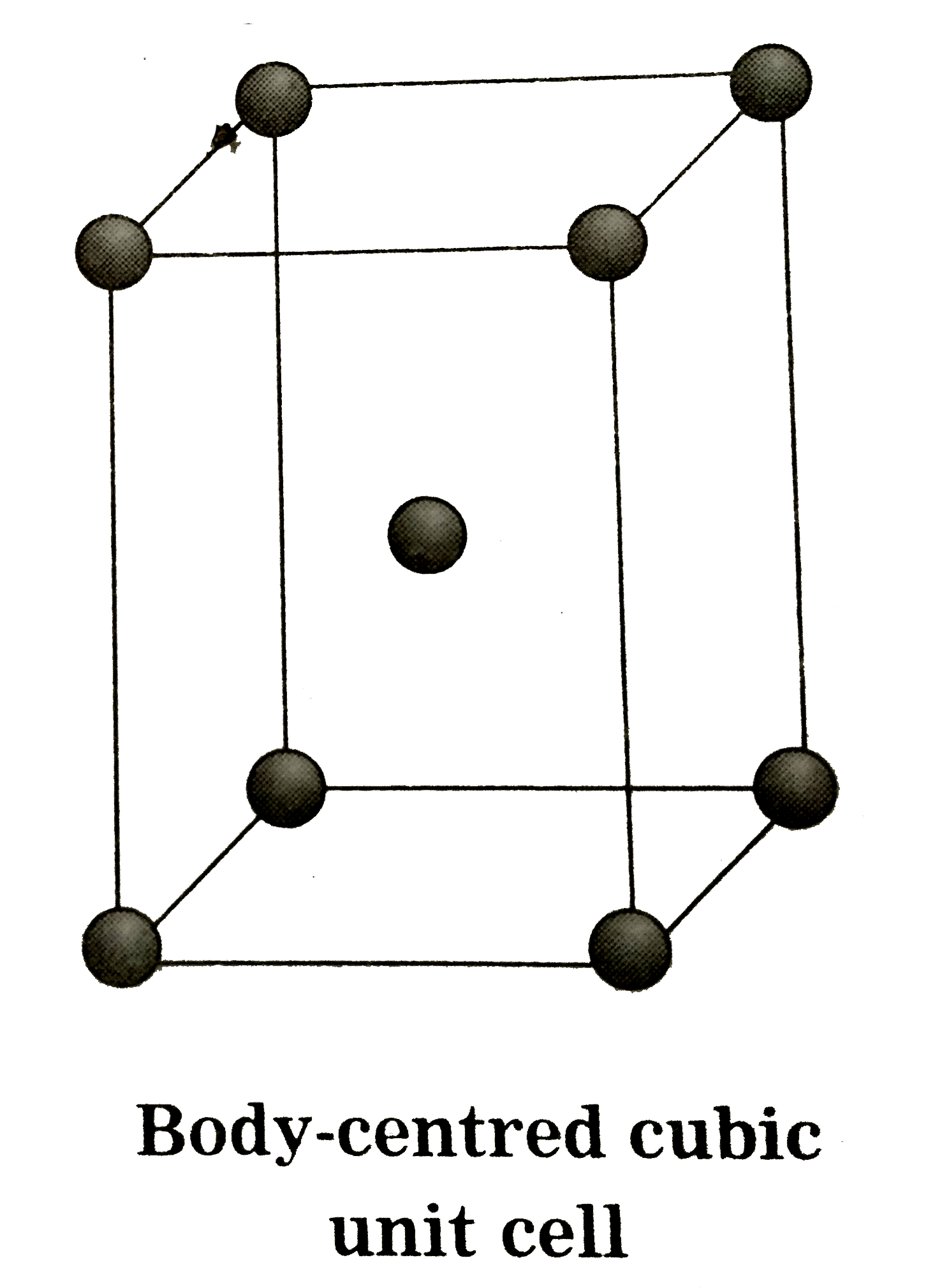
(1) Number of atoms in body-centred cubic (bcc) crystal: In this unit cell, there are 8 atoms at 8 corners and one additional atom at the body centre. Each corner butes 1/8th atom, to the unit cell, hence due to 8 corners.
Number of atoms =8×18
=1 atom.
An atom at the body centre wholly belong to the unit cell.
∴ Total number of atoms present in bcc unit cell =1+1=2
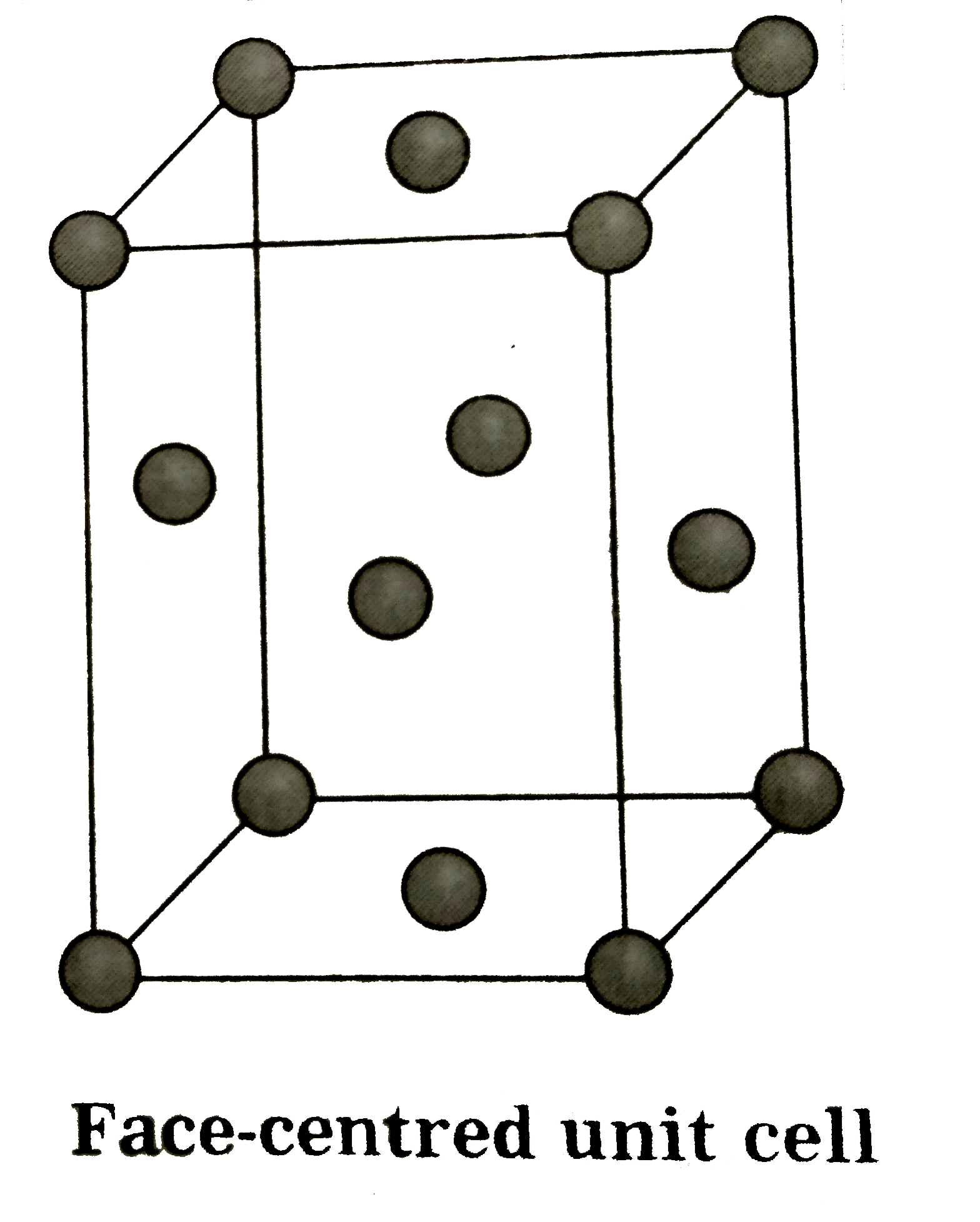
(2) Number of atoms in face-centred cubic (fcc) crystal:
In this unit cell, there are 8 atoms at 8 corners and 6 atoms at 6 face centre.
Each corner contributes 1/8th atom to the unit cell, hence due to 8 corners.
|
Similar Questions
Calculate the number of particles (atoms) per unit cell in a FCC crystal lattice.
View SolutionCrystal of sodium form cubic unit cell with edge length 430pm. Find the number of atoms present per unit cell and also the type of unit cell.
View SolutionThe total number of atoms per unit cell of a face centred cubic crystal is
View SolutionCopper crystals have fcc unit cells. Compute the number of atoms per unit cell of copper crystals
View SolutionCopper crystals have fcc unit cells: Compute the number of atoms per unit cell of copper crystal.
View SolutionFor a crystal of diamond, state :
The number of carbon atoms present per unit cell.
View SolutionWhat is the number of atoms per unit cell in a metallic crystal having BCC structure.
View SolutionIf the number of atoms per unit in a crystal is 2, the structure of crystal is:
View Solution

