The given graph shows the vapour pressure - temperature curves for some liquids
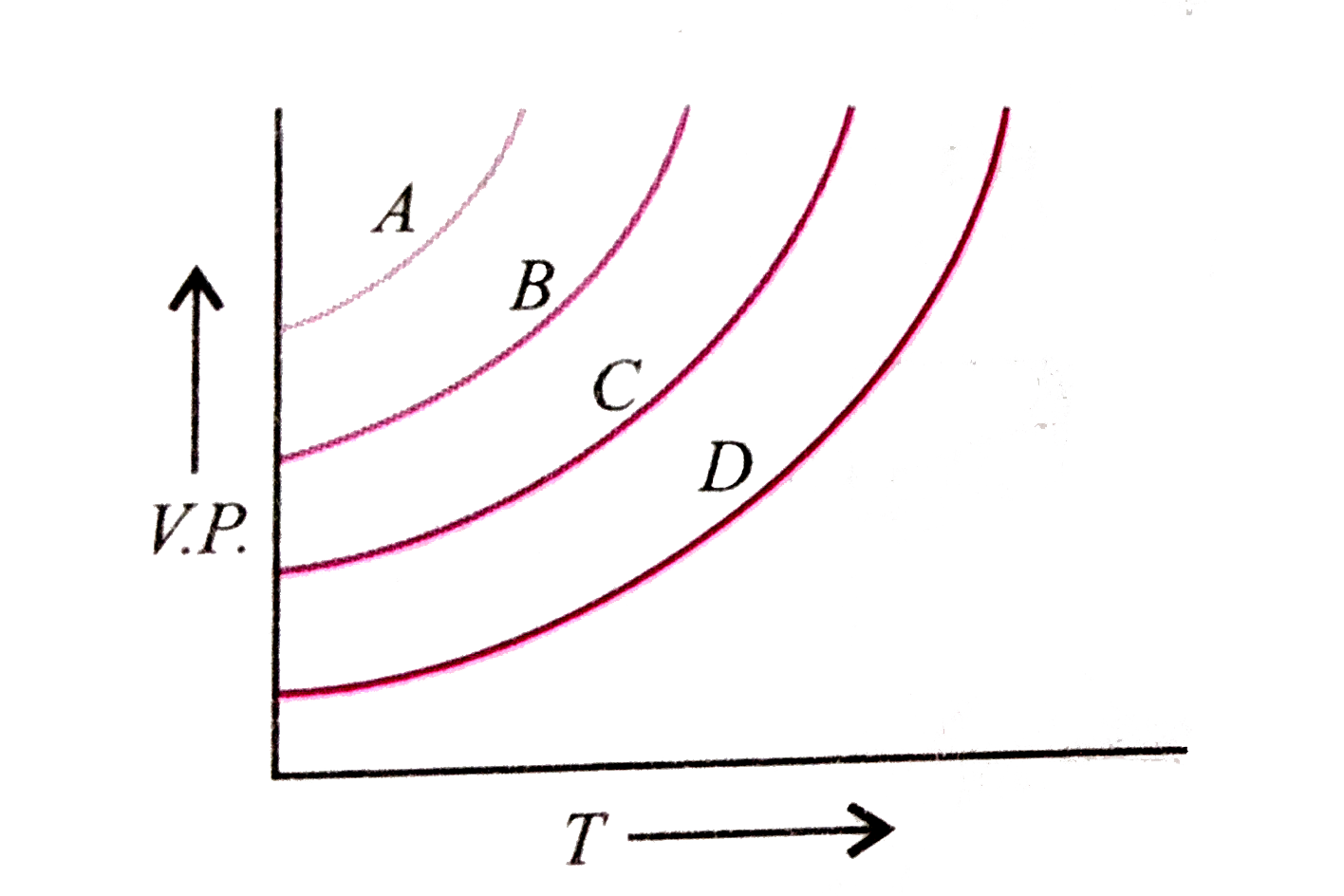
Liquids A,B, C and D respectively are
diethyl ether, acetone , ethyl alcohol , water
acetone , ethyl alcohol, diethyl ether , water
water, ethyl alcohol , acetone , diethyl ether
ethyl alcohol, acetone , diethyl ether, water
The correct Answer is:A
The vapour pressure increases with decrease in intermolecular forces . When the forces are weak, the liquid has high volatility and maximum vapour pressure . Diethyl ether has highest vapour pressure while water has lowest vapour pressure.
Topper's Solved these Questions
- SOLUTIONS BOOK - NCERT FINGERTIPSCHAPTER - SOLUTIONS EXERCISE - Ideal And Non-Ideal Solutions
13 Videos
- SOLUTIONS BOOK - NCERT FINGERTIPSCHAPTER - SOLUTIONS EXERCISE - Colligative Properties And Determination Of Molar Mass
32 Videos
- SOLUTIONS BOOK - NCERT FINGERTIPSCHAPTER - SOLUTIONS EXERCISE - Vapour Pressure Of Liquid Solutions
5 Videos
- PRACTICE PAPER -3BOOK - NCERT FINGERTIPSCHAPTER - PRACTICE PAPER -3EXERCISE - Practice Paper 3
50 Videos
- SURFACE CHEMISTRYBOOK - NCERT FINGERTIPSCHAPTER - SURFACE CHEMISTRYEXERCISE - Assertion And Reason
15 Videos
Similar Questions
For an ideal solution with PA>PB , which of the following is true ?
View SolutionThe relative lowering in vapour pressure is proportional to the ratio of number of
View SolutionPartial pressure of a solution component is directly proportional to its mole fraction. This statement is known as
View SolutionWhich of the following is an addition polymer ?
View SolutionAssertion : Solids in finely divided state act as good adsorbents.
Reason : Adsorption is a surface phenomenon .
View SolutionThe vapour pressure of any liquid shows following change with temperature :-
View Solution The given graph shows the vapour pressure- temperature curves for some liquids.
The given graph shows the vapour pressure- temperature curves for some liquids.
Liquids A,B,C, and D respectively are
View SolutionThe given graph shows the vapour pressure -temperature curves for some liquids.Liquids P,Q,R and S respectively are
View SolutionTwo liquids A and B boil at 125^(@)C and 146^(@)C respectively. Liquid A will have higher vapour pressure.
View SolutionHow many of the following effect the vapour pressure developed in a container ?
View Solutionदिया गया ग्राफ कुछ द्रवों के लिए वाष्प दाब-ताप वक्रों को दर्शाता है।
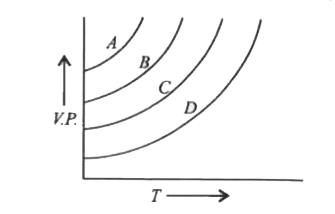
A,B,C एवं D द्रव क्रमशः हैं
View SolutionAs temperature increases, vapour pressure of a liquid
View SolutionAs temperature increases, vapour pressure of a liquid
View SolutionShow graphically the influence of temperature on vapour pressure of a liquid.
View SolutionThe given graph shows the vapour pressure - temperature curves for some liquids

Liquids A,B, C and D respectively are
View SolutionThe vapour pressure of liquid __ with rise of temperature.
View Solution

