Assertion : Sulphide ores are converted to oxides before reduction.
Reason : Oxides are easier to reduce.
If both assertion and reason are true and reason is the correct explanation of assertion .
if both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
The correct Answer is:A
Step by step video, text & image solution for Assertion : Sulphide ores are converted to oxides before reduction. Reason : Oxides are easier to reduce. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBook:NCERT FINGERTIPSChapter:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSExercise:NCERT EXEMPLAR PROBLEMS
Explore 3 Videos
- ELECTROCHEMISTRYBook:NCERT FINGERTIPSChapter:ELECTROCHEMISTRYExercise:Assertion And Reason
Explore 15 Videos
- HALOALKANES AND HALOARENESBook:NCERT FINGERTIPSChapter:HALOALKANES AND HALOARENESExercise:Assertion And Reason
Explore 15 Videos
Similar Questions
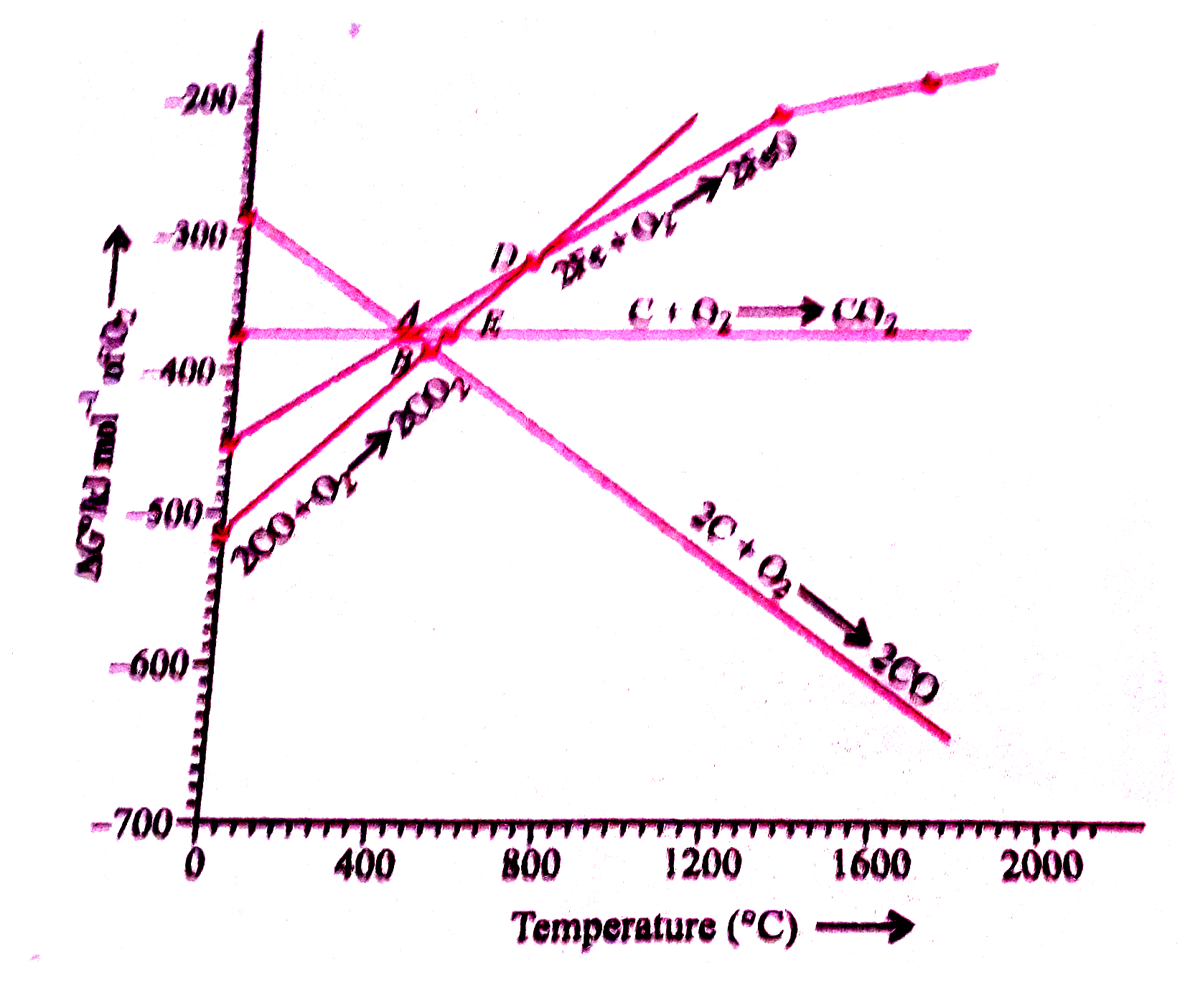
Choose the correct option of temperature at which carbon reduces FeO to iron and produces CO.
View SolutionAssertion:Electrolytic cell uses electrical energy to carry non-spontaneous chemical reactions.
Reason : Chemical energy of a spontaneous redox reaction can be converted into electrical energy.
View SolutionAssertion : CH2=CH−CH2−X is an examples of allyl halides.
Reason: These are the compounds in which the halogen atom is called to an sp2 hybridised carbon atom.
View SolutionWhy are sulphide ores converted to oxide before reduction?
View SolutionWhy are sulphide ores converted to oxide form before reduction to metal ?
View SolutionA sulphide ore is first converted into its oxide before reduction. This is done because :
View SolutionAssertion : Ores are generally converted into oxides, prior to reduction. Reason : Metal oxides can be easily reduced.
View SolutionExplain - Sulphide ores are generally converted into oxides before reduction.
View SolutionWhy are sulphide ores converted to oxide form before reduction to metal?
View SolutionA sulphide ore is first converted into its oxide before reduction. This is done because :
View SolutionWhy are sulphide ores converted to oxide before reduction?
View Solutionअभिकथनः सल्फाइड अयस्कों को अपचयन के पूर्व ऑक्साइडों में परिवर्तित किया जाता है।
तर्क: ऑक्साइडों को अपचयित करना आसान होता है।
View SolutionWhy are sulphide ores converted to oxide before reduction ?
View SolutionWhy are sulphide ores converted to oxide before reduction?
View Solutionकथन : अपचयन से पहले अयस्क सामान्यतया आक्साइड में परिवर्तित किये जाते है।
कारण : धातु आक्साइड आसानी से अपचयित हो सकते है।
View Solution
