The decreasing order of boiling points of the following hydrides is
A
SbH3>AsH3>PH3>NH3
B
NH3>SbH3>AsH3>PH3
C
SbH3>NH3>AsH3>PH3
D
PH3>AsH3>SbH3>NH3
Video Solution
Text Solution
The correct Answer is:C
NH3 has abnormally high boiling points because of their tendency to form hydrogen bonds. NH3 has higher boiling point than phosphine and then boiling point increases down the group because of increase in size. Hence, the order of boiling point will be SbH3>NH3>AsH3>PH3
|
Topper's Solved these Questions
- THE P-BLOCK ELEMENTS Book:NCERT FINGERTIPSChapter:THE P-BLOCK ELEMENTS Exercise:Dinitrogen
Explore 4 Videos
- THE P-BLOCK ELEMENTS Book:NCERT FINGERTIPSChapter:THE P-BLOCK ELEMENTS Exercise:Ammonia
Explore 4 Videos
- THE D- AND F- BLOCK ELEMENTS Book:NCERT FINGERTIPSChapter:THE D- AND F- BLOCK ELEMENTS Exercise:Assertion And Reason
Explore 15 Videos
- THE SOLID STATE Book:NCERT FINGERTIPSChapter:THE SOLID STATE Exercise:Assertion And Reason
Explore 15 Videos
Similar Questions
Knowledge Check
The decreasing order of boiling points is
An−pentane>iso−pentane>≠opentaneBIso−pentane>pentane>≠o−pentaneCNeo−pentane>iso−pentane>n−pentaneDn−pentan>≠opentaneiso−pentaneThe decreasing order of boiling points is
Aneo-pentane > iso-pentane > n-pentaneBiso-pentane > n-pentane > neo-pentaneCn-pentane > iso-pentane > neo-pentaneDn-pentane > neo-pentane > iso-pentane.The decreasing order of the boiling points of the following hydrides
(i) NH3 (ii) PH3
(iii) AsH3 (iv) SbH3
(v) H2O isA(v)>(iv)>(i)>(iii)>(ii)B(v)>(i)>(ii)>(iii)>(iv)C(ii)>(iv)>(iii)>(i)>(v)D(iv)>(iii)>(i)>(ii)>(v)
The decreasing order of boiling points of the following compounds is:
(I)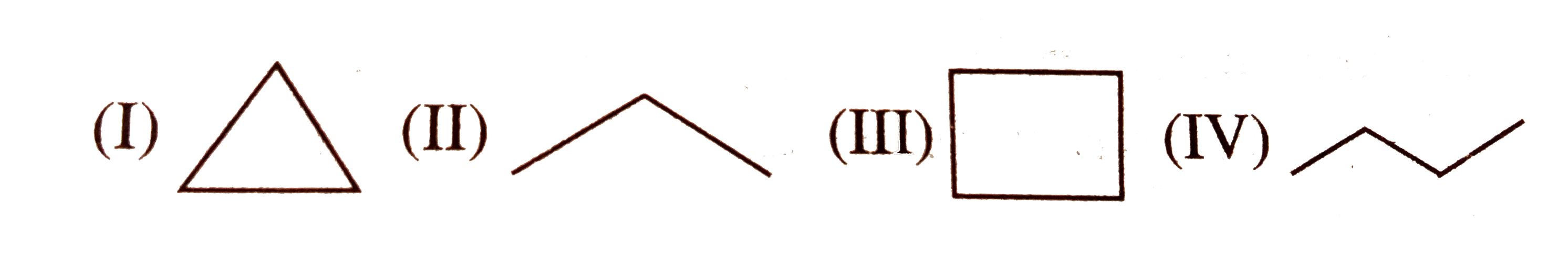
View SolutionBoiling points of the following order :
View SolutionThe correct order of boiling point among the following.
View SolutionWhich is in the decreasing order of boiling points of V group hydrides ?
View SolutionWhat is decreasing order of Boiling point
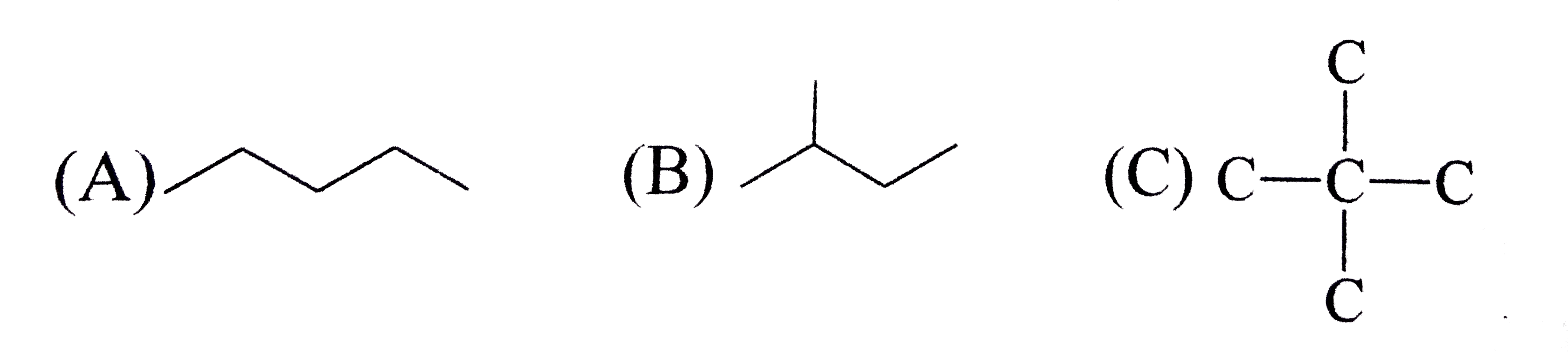
View Solution
