The correct order of acidity of oxoacids of halogens is
A
HCIO<HCIO2<HClO3<HClO4
B
HClO4<HCIO3<HClO2<HCIO
C
HCIO<HCIO4<HClO3<HCIO2
D
HClO4<HClO2<HClO3<HCIO
Video Solution
Text Solution
The correct Answer is:A
Acidic strength of oxoacids of a particular halogen atom increases with increase in oxidation number thus the order of acidic strength is
+1HClO<+3HClO2<+5HClO3<+7HClO4
|
Similar Questions
Knowledge Check
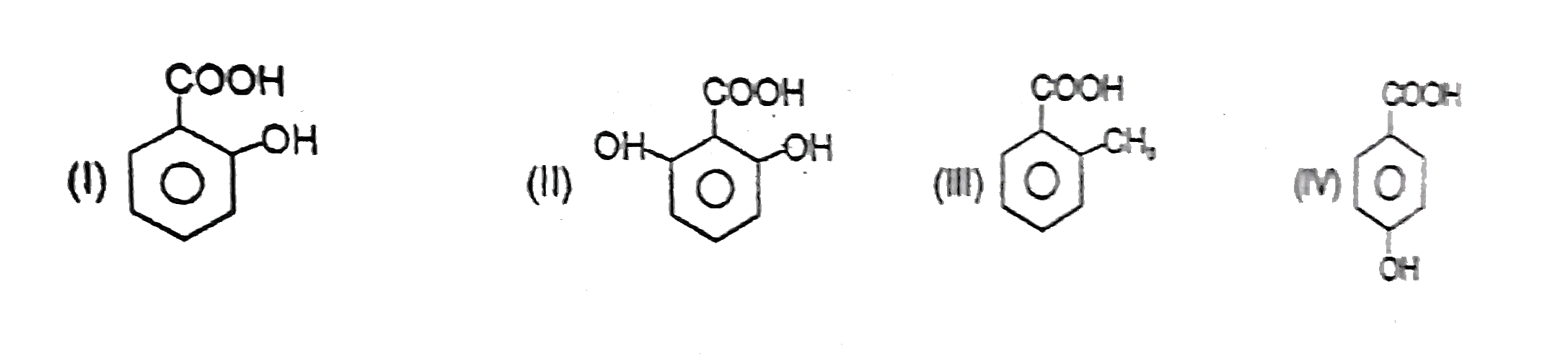
Correct order of acidity is:
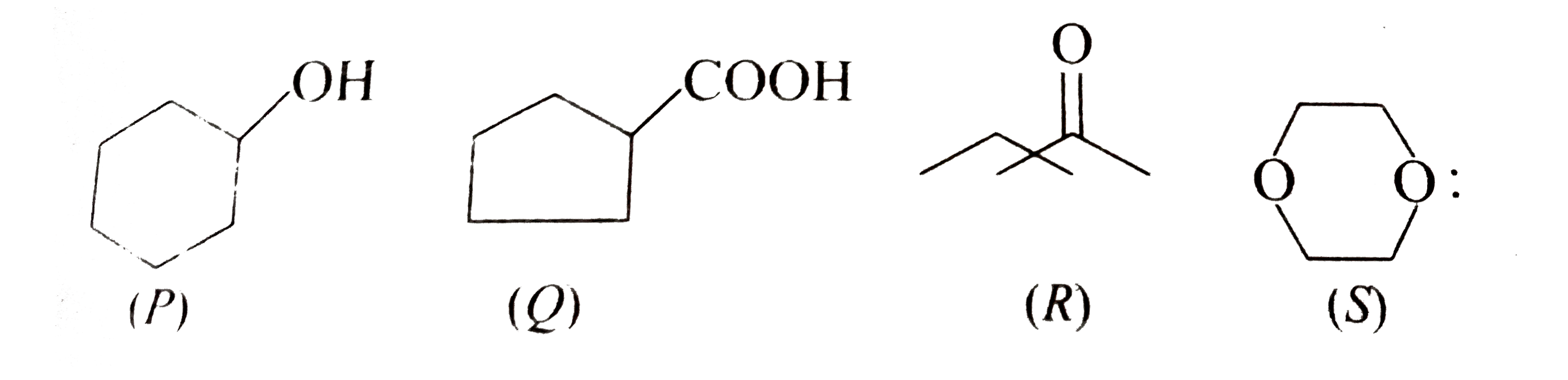 AP>Q>S>RBQ>R>S>PCQ>P>R>SDR>P>Q>S
AP>Q>S>RBQ>R>S>PCQ>P>R>SDR>P>Q>SThe correct order of acidic character is
AP4O10>P4O6BN2O5>N2O3CN2O3>P4O6DAll of the aboveWrite the correct order of acidity :
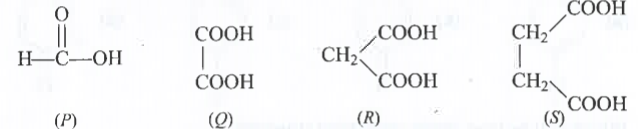 AP>Q>R>SBQ>P>R>SCQ>R>S>PDS>R>Q>P
AP>Q>R>SBQ>P>R>SCQ>R>S>PDS>R>Q>P