In water molecule, the two O - H bonds are oriented at an angle of 104.5∘ . In BF3, the three B - F bonds are oriented at an angle of 120∘ . In BeF2, the two Be - F bonds are oriented at an angle of 180∘. Which of the following will have highest dipole moment ?
BeF2
BF3
H2O
All have zero dipole moment.
The correct Answer is:C
Topper's Solved these Questions
- CHEMICAL BONDING & MOLECULAR STRUCTUREBook:NCERT FINGERTIPSChapter:CHEMICAL BONDING & MOLECULAR STRUCTUREExercise:MCQs
Explore 4 Videos
- CHEMICAL BONDING & MOLECULAR STRUCTUREBook:NCERT FINGERTIPSChapter:CHEMICAL BONDING & MOLECULAR STRUCTUREExercise:Vsepr Theory
Explore 15 Videos
- CHEMICAL BONDING & MOLECULAR STRUCTUREBook:NCERT FINGERTIPSChapter:CHEMICAL BONDING & MOLECULAR STRUCTUREExercise:Ionic And Electrovalent Bond
Explore 4 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY Book:NCERT FINGERTIPSChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY Exercise:Assertion And Reason
Explore 15 Videos
Similar Questions
What is the formal charge on carbon atom in the following two structures :
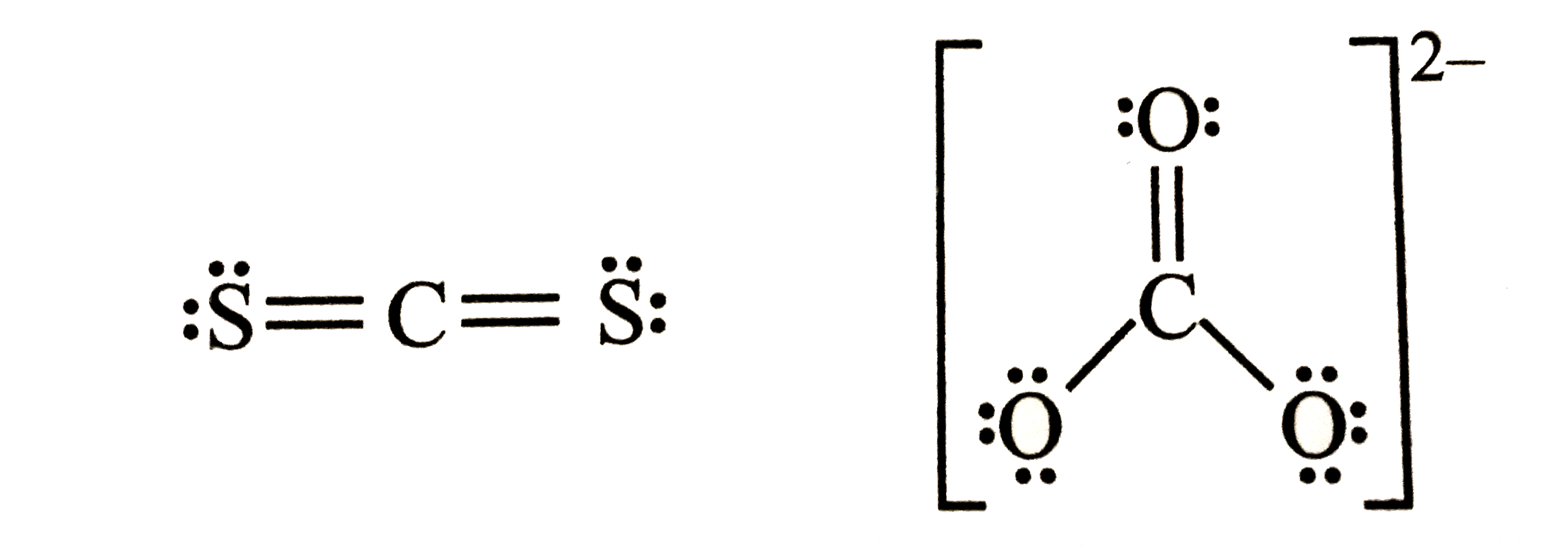
View SolutionVSEPR THEORY
View SolutionTwo elements X and Y combine to form a compound XY. Under what conditions the bond formed between them will be ionic ?
View SolutionAssertion:According to mendeleev,the properties of elements are a periodic function of their atomic weights.
Reason:Mendeleev left the gap under aluminium and a gap under silicon,and called these elements Eka-Aluminium and Eka-silicon.
View SolutionThe experimental dipole moment of water molecule is 1.84D . Calculater the bond angle H-O-H in water molecule if dispole moment of OH bond is 1.5D .
View SolutionIn water molecule, the O-H bonds are oriented
at an angle of 104.5∘. In BF3 , the three B-F bonds
are oriented at an angle of 120∘ . InBeF2 , the two
Be-F bonds are oriented at an angle of 180∘. Which
of the following will have highest dipole moment?
View SolutionThe bond angle of water is 104.5∘ due to ________.
View Solutionजल के अणु में H - O - H बन्ध कोण लगभग 180° होता है।
View SolutionCovalent bonds have definite orientations but electrovalent bonds have no definite orientation-explain.
View Solutionजल का बंध कोण 104.5∘ है, इसका कारण है
View Solutionपानी के अणु में, दो O - H बंध 104.5∘ के कोण पर अभिविन्यस्त रहते हैं। BF3 में, तीन B- Fबंध 120∘ के कोण पर अभिविन्यस्त रहते हैं। BeF2 , में, दो Be-F बंध 180∘ के कोण पर अभिविन्यस्त रहते हैं। निम्न में से किसका द्विध्रव आपूर्ण उच्चतम होगा?
View SolutionIn water molecule, the two O - H bonds are oriented at an angle of 104.5∘ . In BF3, the three B - F bonds are oriented at an angle of 120∘ . In BeF2, the two Be - F bonds are oriented at an angle of 180∘. Which of the following will have highest dipole moment ?
View SolutionThe bond angle of water is 104.5^(@) due to ________.
View Solutionनिम्नलिखित में से किस यौगिक में दो सहसंयोजी बन्ध के मध्य आबन्ध कोण सर्वाधिक है?
View Solutionनिम्नलिखित में से किस यौगिक में दो सहसंयोजी बन्ध के मध्य आबन्ध कोण सर्वाधिक है?
View Solution

