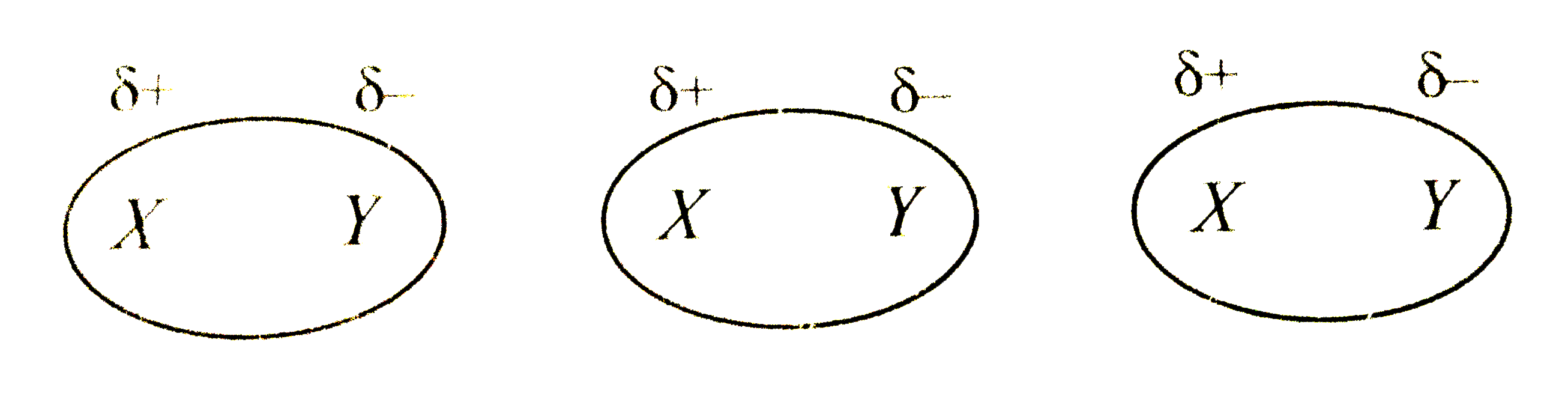
Study the figure given below and mark the correct expression.
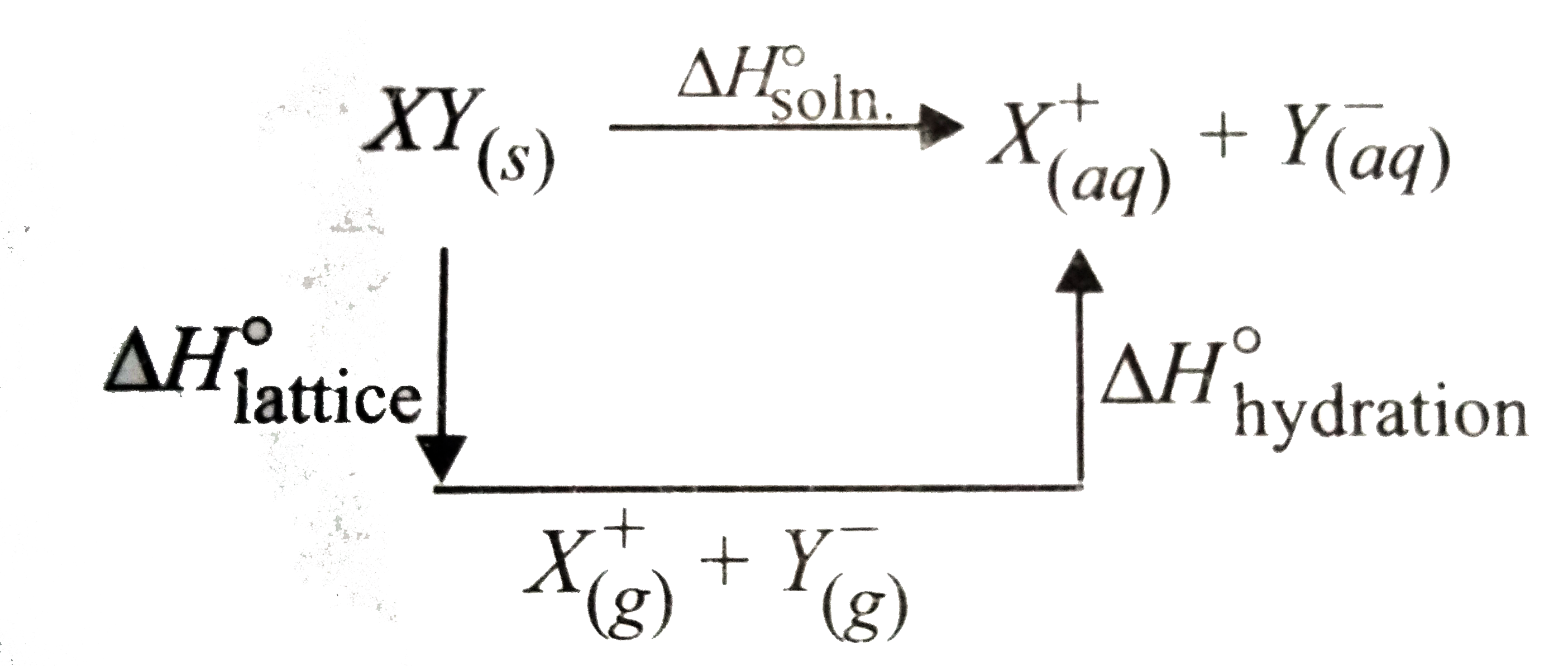
The enthalpy of solution of XY(s),ΔH∘soln. in water can be determined by
ΔH∘lattice=ΔH∘hyd+ΔH∘Soln.
ΔH∘hyd=ΔH∘lattice+ΔH∘soln.
ΔH∘Soln.=ΔH∘lattice+ΔH∘hyd
ΔH∘soln=ΔH∘lattice×ΔH∘hyd
The correct Answer is:C
Step by step video & image solution for Study the figure given below and mark the correct expression. The enthalpy of solution of XY_((s)),DeltaH_("soln.")^(@) in water can be determined by by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Study the given figure and choose the correct option -
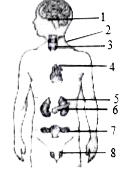 A2.→ Parathormone
A2.→ Parathormone
6.→ islets of langerhans
8.→ ICSH production
3.→ ThyroxineB5.→ Adrenaline
1. → FSH production 8.→ Testosterone
4.→ ImmunityC1.→ Master gland
8.→ ICSH production 6.→ Thyroxine
5.→ AdrenalineD7.→ LH production
8.→ Testosterone
6.→ Insulin
4.→ Thyroxine
Study the above given figure and choose the correct match:-
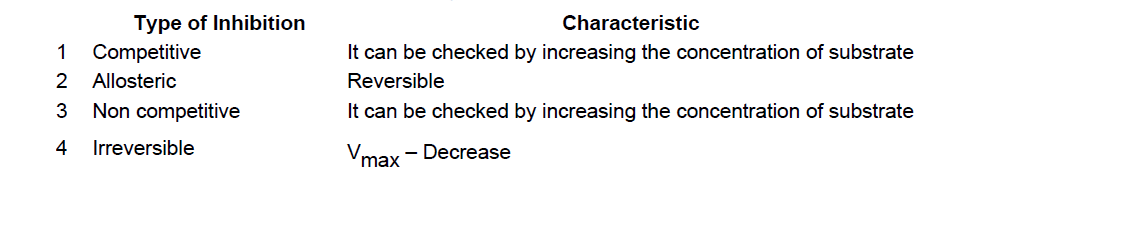 A1B2C3D4
A1B2C3D4
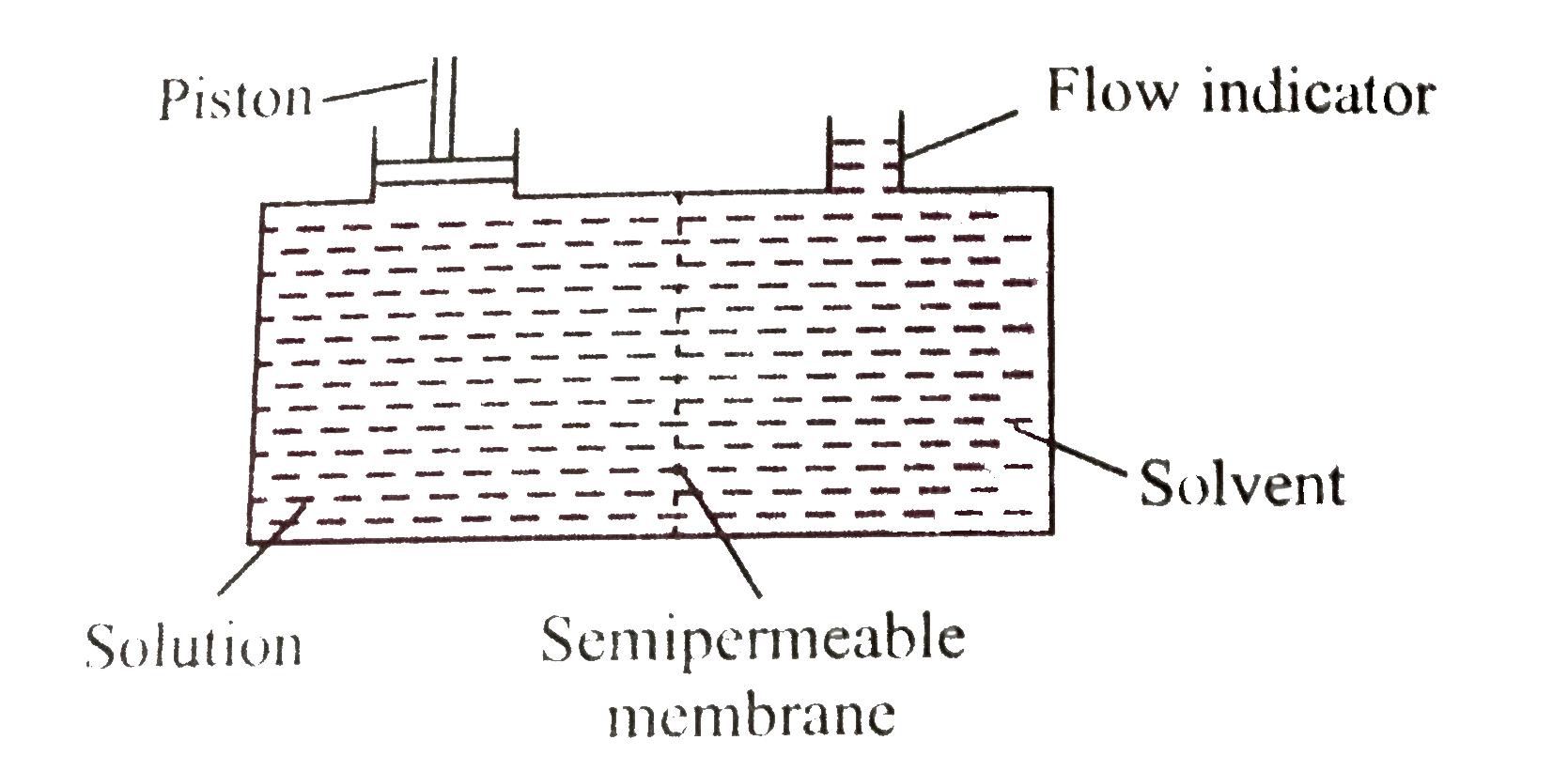
Study the following figure showing osmosis and mark the correct statement .
View SolutionStudy the figure below and mark the correct statement about Kc and dependence of extent of reaction on it.
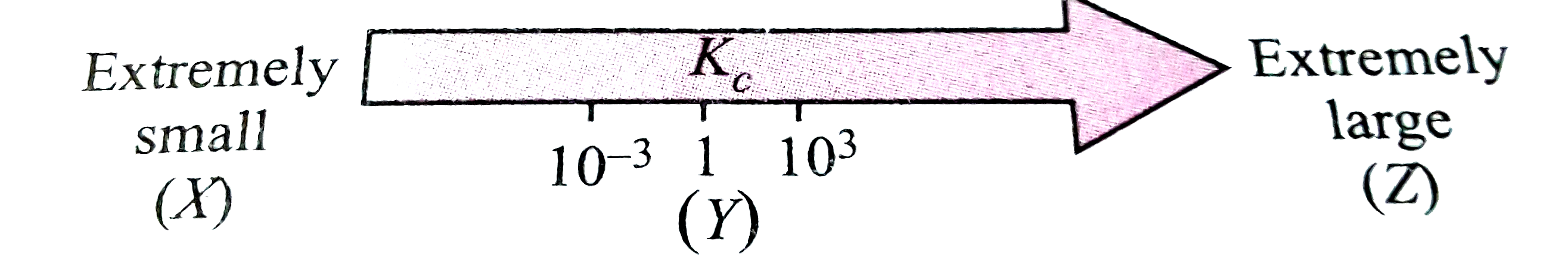
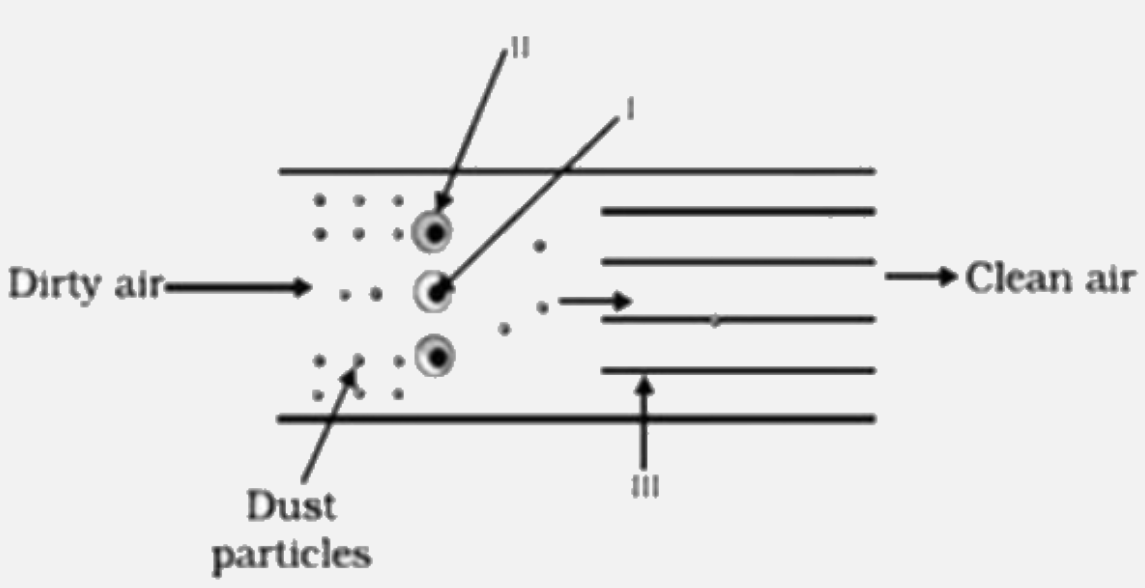
View SolutionStudy the diagram given below and mark the INCORRECT statement related to this .
View SolutionStudy the figure given below and choose the option that repersent correct exaple of ech phyllotxy

View SolutionStudy the figures below and identify the type of interaction between XY-XY molecules.
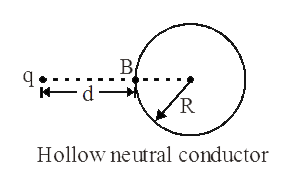
View SolutionFor the situation shown in the figure below, mark out the correct statement(s) :
View Solution