Which of the following is a decreasing order of oxidation states of the central atoms?
A
PCl5,HIO4,Cl2O2−7,Cl2O
B
Cr2O2−7,Cl2O,HIO4,PCl5
C
HIO4,Cr2O2−7,PCl5,Cl2O
D
Cr2O2−7,HIO4,Cl2O,PCl5
Video Solution
Text Solution
The correct Answer is:C
Cl2O→2x+1(−2)=0⇒x=+1
Cr2O2−7→2x+7(−2)=−2⇒x=+6
HIO4→+1+x+4(−2)=0⇒x=+7
PCl5→x+(−5)=0⇒x=+5
|
Similar Questions
Knowledge Check
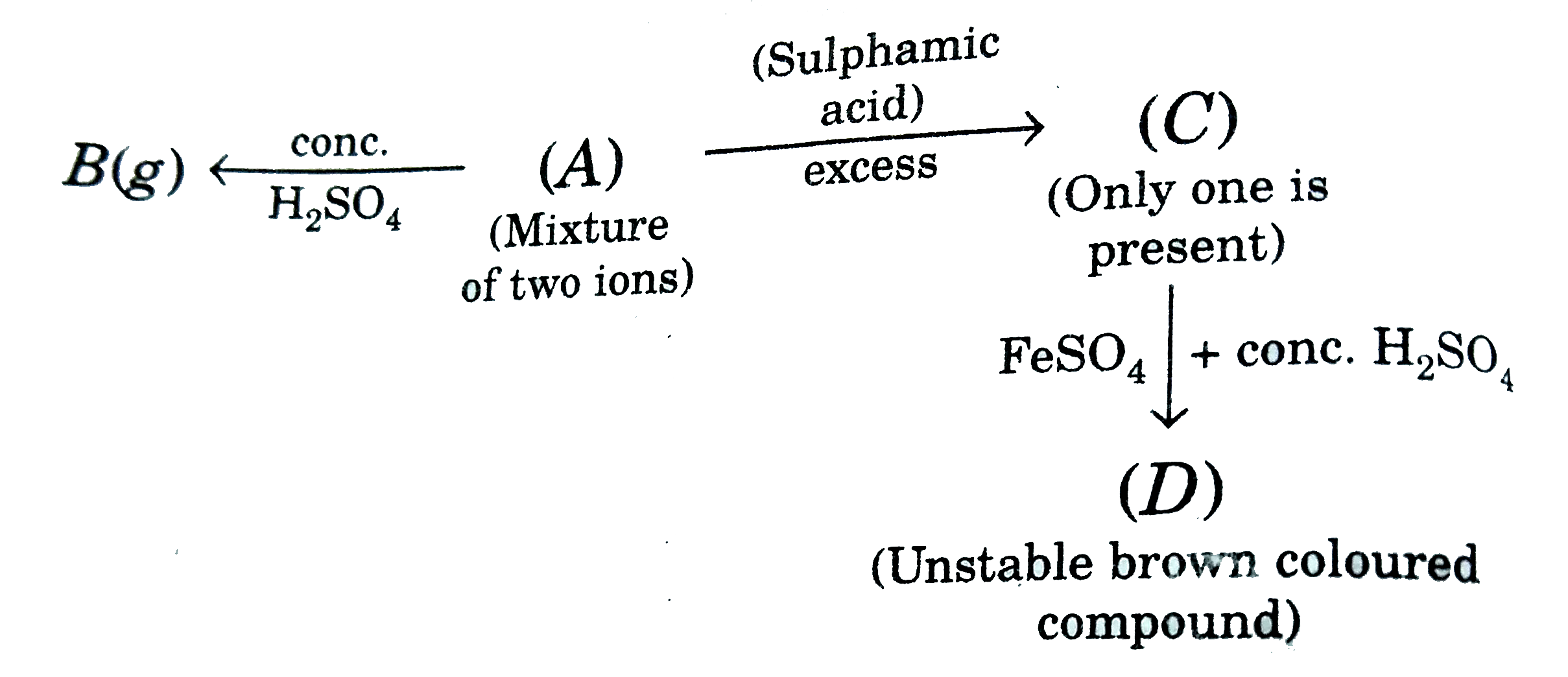
What is oxidation state of central atom of (D)?A+3B+2C+1DZeroWhich of the following complex has same oxidation state of the central metall atom in the cationic and anionic part ?
A[Pt(py)4][PtCl4]B[Pt(NH3)4][PtCl6]C[Pt(NH3)4Cl2][PtCl4]DK4[Ni(CN)6]Which of the following is the CORRECT decreasing order of the stabilities of the following metal oxides on the basis of Ellingham diagram ?
AAl2O3>Cr2O3>MgO>Ag2O>FeOBMgO>Al2O3>Cr2O3>FeO>Ag2OCCr2O3>AgO>Al2O3>MgO>FeODAg2O>FeO>Cr2O3>Al2O3>MgO
Which of the following have been arranged in the decreasing order of oxidation number of sulphur ?
View SolutionWhich of the following molecule have least oxidation state of the nitrogen atoms ?
View SolutionThe hybridization state of the central atom in HgCl2 is
View SolutionIn which of the following pairs of compounds, the hybrid state of the central atom is same?
View SolutionWhich order of the compounds is according to the decreasing order of oxidation state of nitrogen
View Solution
