What is the trend of boiling points of hydrides of N, O and F ?
Due to lower molecular masses NH3,H2O and HF have lower boiling points than those of the subsequent group member hydrides.
Due to higher electronegativity of N, O and F, NH3,H2O and HF show hydrogen bonding and hence higher boiling points than the hydrides of their subsequent group members.
There is no regular trend in the boiling points of hydrides.
Due to higher oxidation states of N, O and F, the boiling points of NH3,H2O and HF are higher than the hydrides of their subsequent group members.
The correct Answer is:B
Due to higher electronegativity, the extent of hydrogen bonding is quite appreaciable.
Similar Questions
Knowledge Check
What is decreasing order of Boiling point
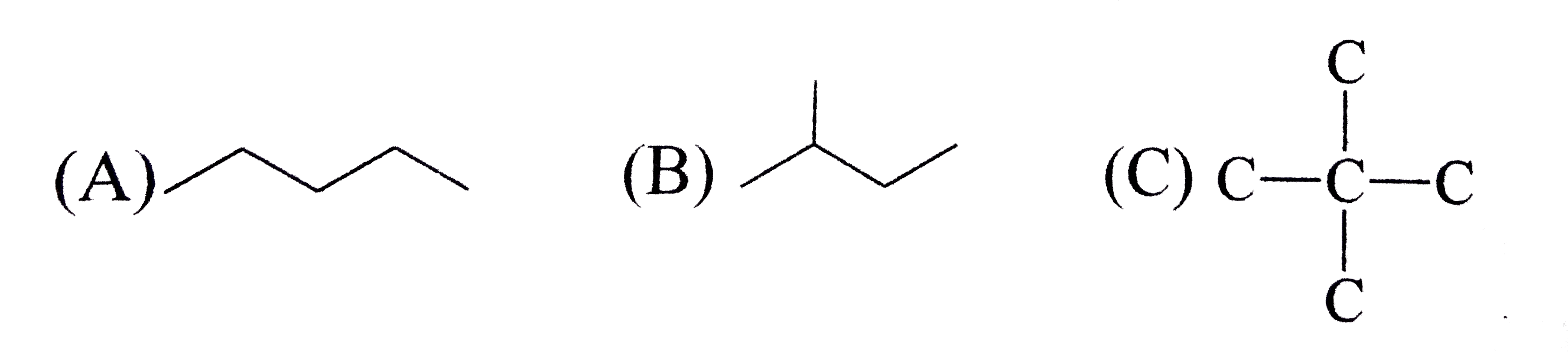 AA>B>CBB>C>ACA>C>BDC>B>A
AA>B>CBB>C>ACA>C>BDC>B>AThe boiling points of hydrides of group 16 are in the order
AH2O>H2Te>H2S>H2SeBH2O>H2S>H2Se>H2TeCH2O>H2Te>H2Se>H2SDH2O>H2Se>H2S>H2O.Which is in the decreasing order of boiling points of V group hydrides ?
ANH3>PH3>AsH3>SbH3BSbH3>AsH3>PH3>NH3CPH3>NH3>AsH3>SbH3DSbH3>NH3>AsH3>PH3
what is boiling point ?
View Solutionwhat is boiling point ?
View SolutionWhat is meant by boiling point?
View SolutionGive correct order of boiling point of hydride of group 17.
View SolutionFifth group elements form hydrides to type AH3. The hydrides have a lone pair of electrons. The hydries are reducing in nature and the reducing power is related to the stability of A-H bonds. The hydrides are covalent and low boiling. Their boiling points depends on their ability to from hydrogen bond and their molecular size which decide the intermolcular forces in the hydrides .
The boiling points of the hydrides of V-group elements are in the order :
View Solution
