Electrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
H3C−HC=CH2+H+→?
2∘ carbanion
1∘ carbocation
2∘ carbocation
1∘ carbanion
The correct Answer is:C
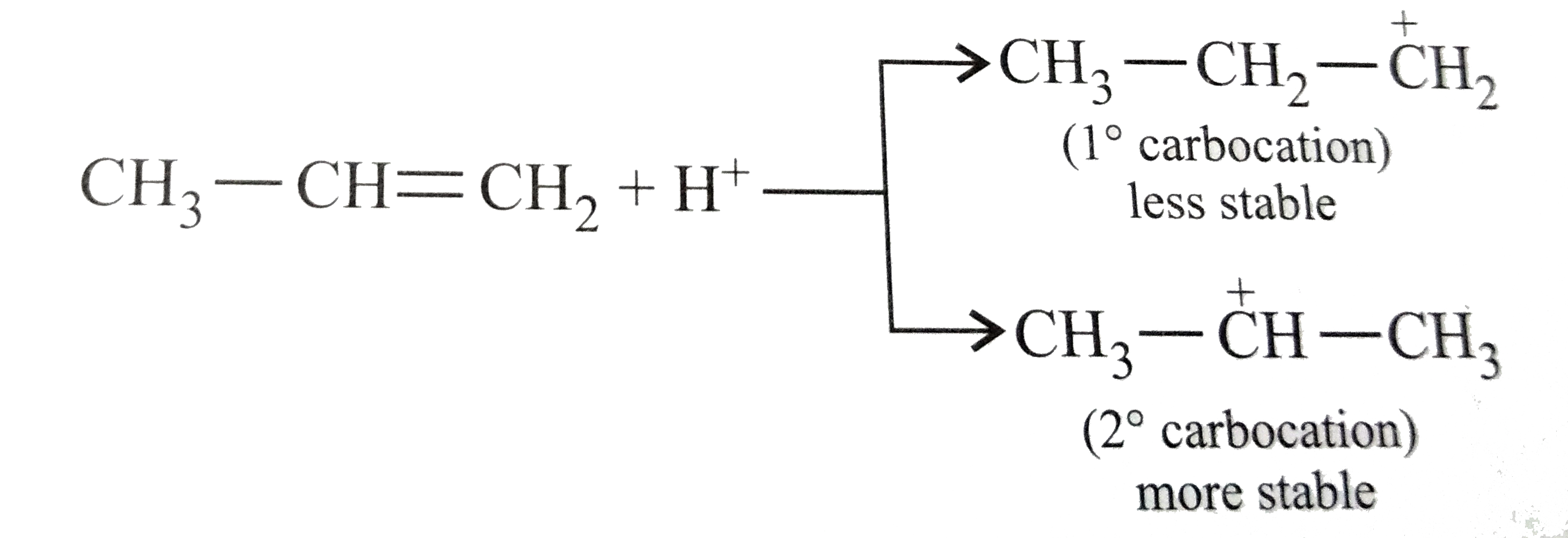
Thus, 2∘ carbocations if formed.
Topper's Solved these Questions
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES BOOK - NCERT FINGERTIPSCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES EXERCISE - Exemplar Problems
1 Video
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES BOOK - NCERT FINGERTIPSCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES EXERCISE - Assertion And Reason
12 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES BOOK - NCERT FINGERTIPSCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES EXERCISE - Higher Order Thinking Skills
6 Videos
- HYDROGEN BOOK - NCERT FINGERTIPSCHAPTER - HYDROGEN EXERCISE - Assertion And Reason
15 Videos
- PRACTICE PAPER 1BOOK - NCERT FINGERTIPSCHAPTER - PRACTICE PAPER 1EXERCISE - Practice Paper 1
44 Videos
Similar Questions
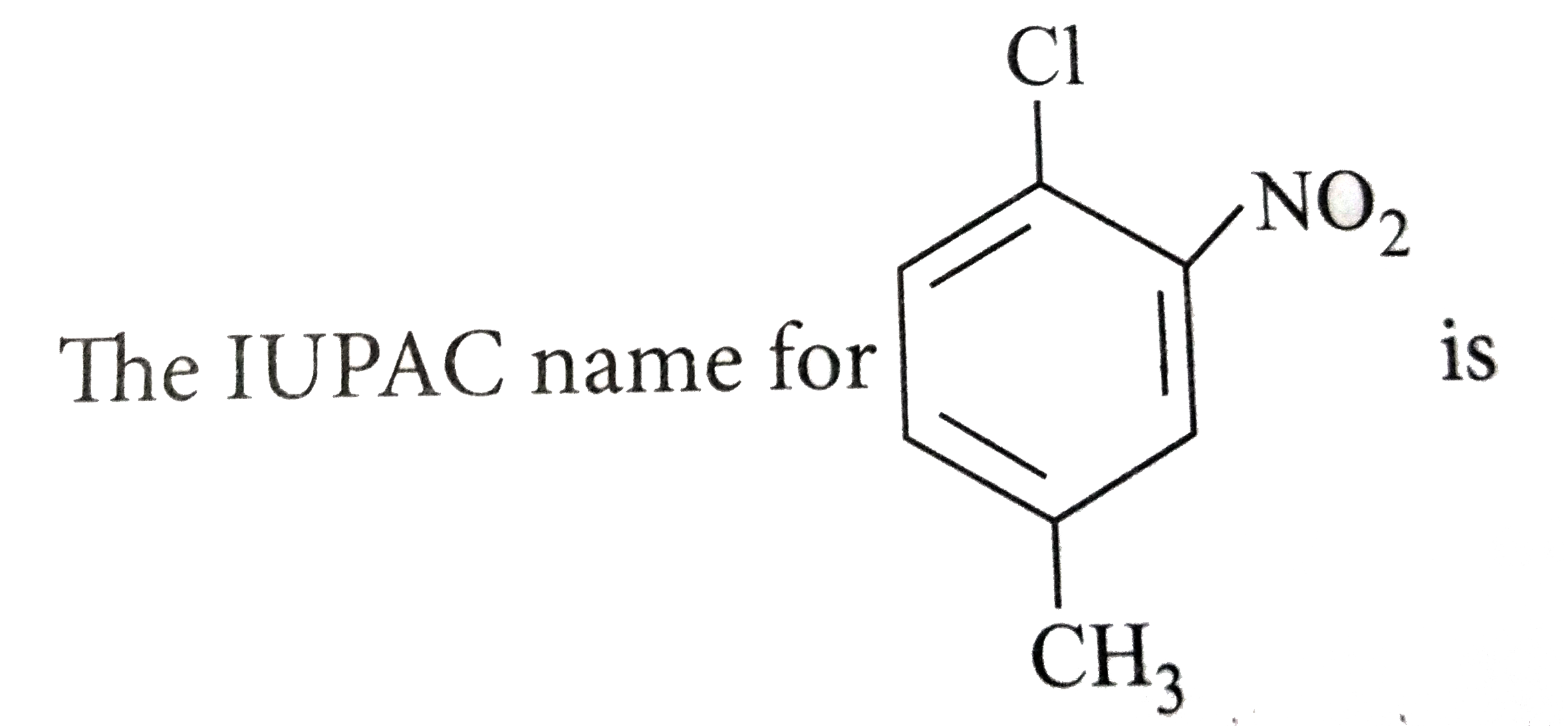
The IUPAC name for
View SolutionAssertion: Hybridisation influences the bond length and bond enthalpy in organic compound.
Reason: More the s character of hybrid orbital, shorter and stornger will be the bond.
View SolutionConsider the following reactions.
I. CH3=CHCOOHΔ−−→CH2=CH3
II.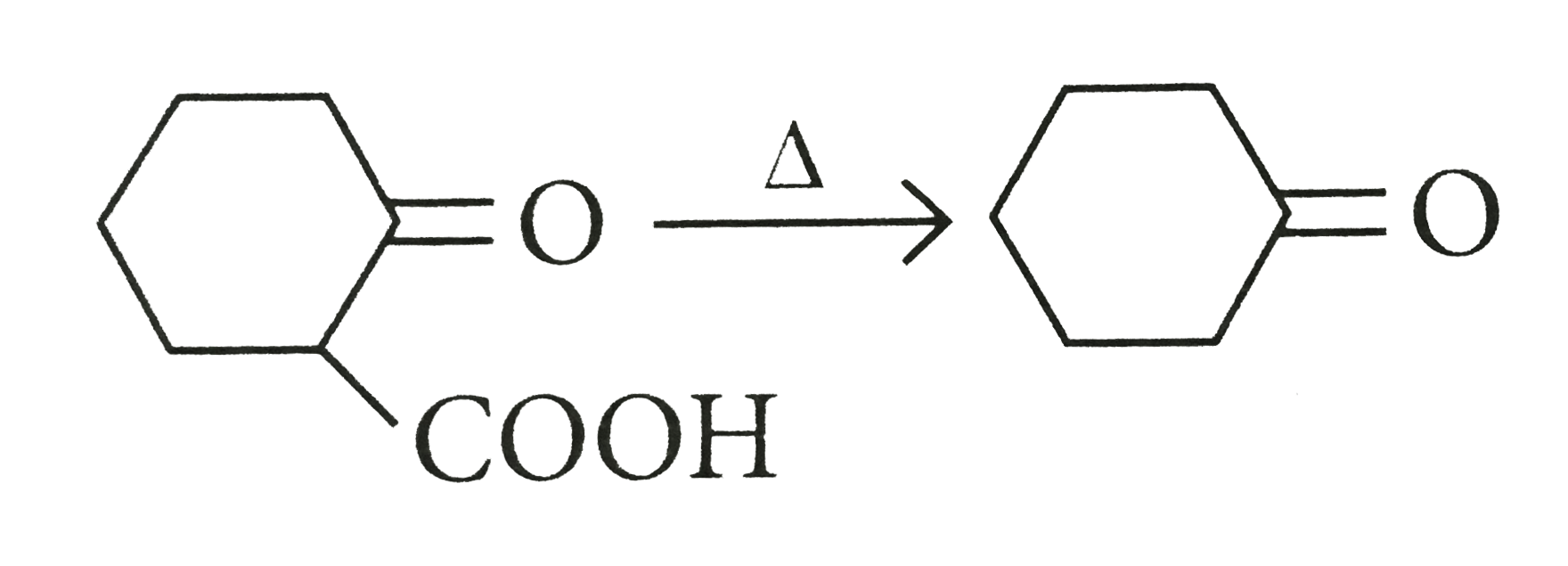
III. CH3CH(COOH)2Δ−−→CH3CH2COOH
In which cases, parent compound loses its functional group in preference?
View SolutionAssertion : In atomic form hydrogen consists of one proton and one electron.
Reason : In elemental form hydrogen exists as a diatomic molecule and is called dihydrogen.
View SolutionThe equilibrium constant at 717 K for the reaction: H2(g)+I2(g)⇐2HI(g) is 50.
The equilibrium constant for the reaction:
2HI2(g)⇐H2(g)+I2(g) is
View SolutionAlkenes undergo electrophilic addition reactions. Electrophilic addition to unsymmetrical alkenes involve the formation of a more stable carbocation intermediate in the first step which is rate determining and carbocation is then attacked by a nuclephile to form product
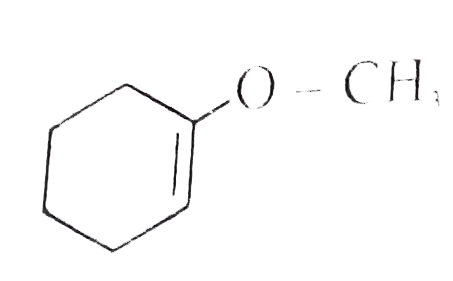 +H2OH2SO4−−−→ Product
+H2OH2SO4−−−→ Product
View SolutionElectrophillic addition reaction proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
H2C−HC=CH2+H+→ ?
View SolutionElectrophilic additions reactions proceed in two steps. The first step involves the additions of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction H_(3)C - HC = CH_(2) + H^(+) rarr ?
View SolutionElectrophilic additions reactions proceed in two steps. The first step involves the additions of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction H3C−HC=CH2+H+→?
View SolutionAssertion (A): addition of HCN in alkene is a type of electrophilic substitution reaction. Reason (R) : in first step , H_(3)O^(+) attacks on double bond .
View SolutionElectrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate reaction. H_(3)C-HC=CH_(2)+H^(+)to?
View SolutionElectrophilic additions reactions proceed in two steps. The first step involves the additions of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction H_(3)C - HC = CH_(2) + H^(+) rarr ?
View SolutionElectrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
H3C−HC=CH2+H+→?
View SolutionElectrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name of type of intermediate formed in the first step of the following addition reaction . CH_(3)-CH= CH_(2) + H^(+) rarr ?
View SolutionElectrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name of type of intermediate formed in the first step of the following addition reaction . CH_(3)-CH= CH_(2) + H^(+) rarr ?
View Solution

