Mark out the correct increasing order of radius.
A
As3−<Br−<K+<Mg2+
B
Mg2+<K+<Br−<As3−
C
Mg2+<K+<As3−<Br−
D
K+<Mg2+<Br−<As3−
Text Solution
The correct Answer is:B
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Mark out the correct options
ADiamagnetism does not occur in all materialsBDiamagnetism results from the partial alignment of permanent magnetic moment.CThe magnetising field intensity is always zero in free space.DThe magnetic field of induced magnetic moment is opposite to the applied field.Mark out the correct options
ADiamagnetism does not occur in all materialsBDiamagnetism results from the partial alignment of permanent magnetic moment.CThe magnetising field intensity is always zero in free space.DThe magnetic field of induced magnetic moment is opposite to the applied field.Mark out the correct decreasing order of ease of addition of halogen.
A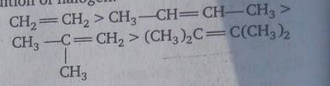 B
B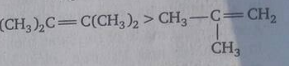 C
C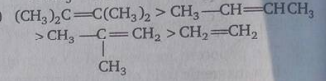 D
D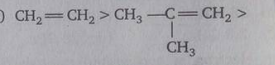
Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
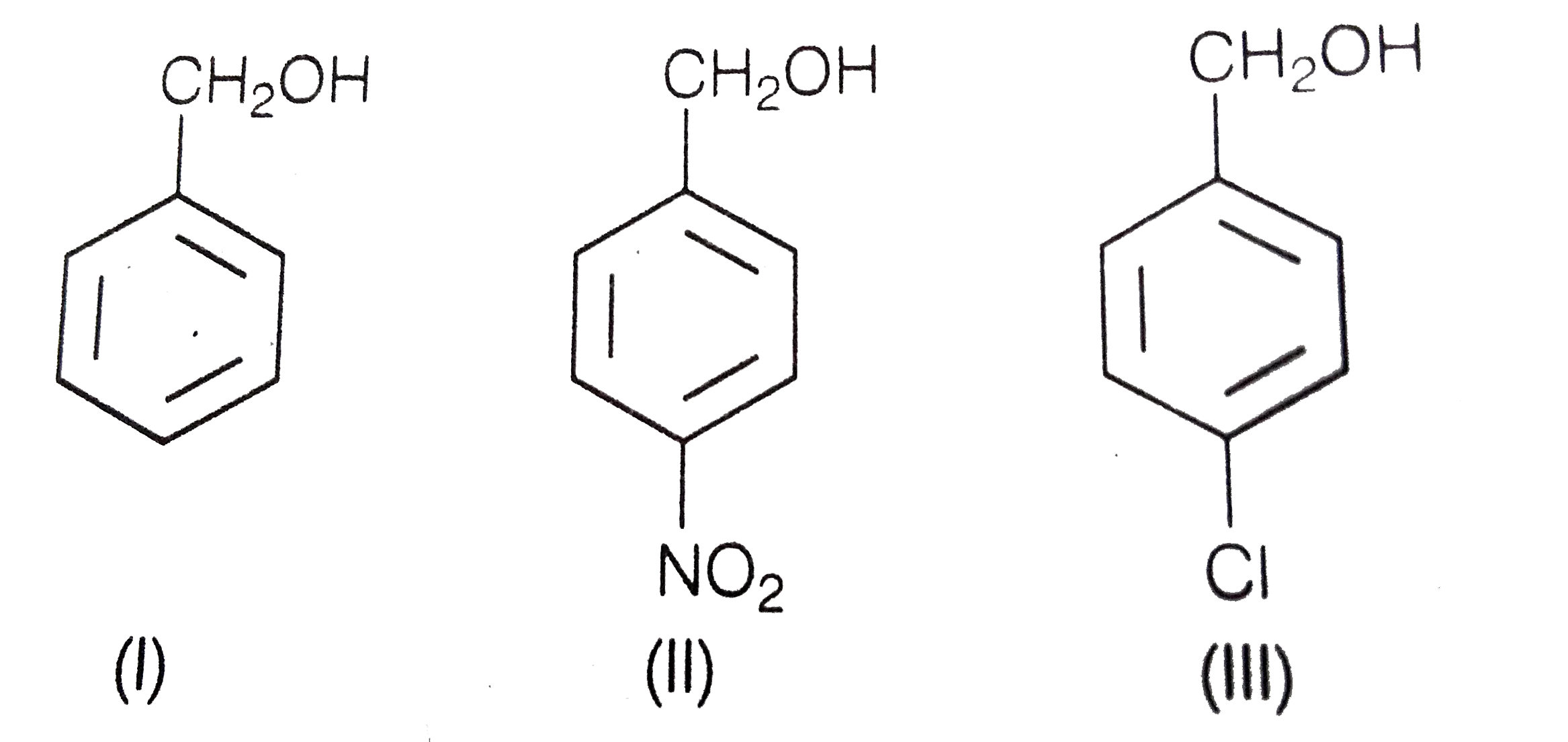
View SolutionMark the correct increasing order of reactivity of the following compounds with HBr/HCl.
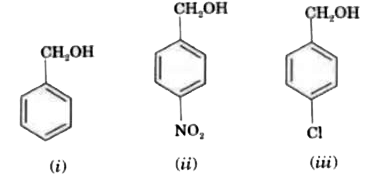
View SolutionMark out the correct options:
View SolutionMark out the correct options.
View SolutionMark out the correct options.
View Solution

