what is the correct IUPAC name of the given compound? 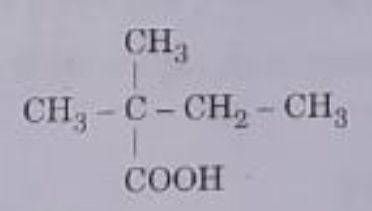
(a) 2,2-Dimethylbutanoic acid
(b) 2-Carboxyl-2-methylbutane
(c) 2- Ethyl-2-methylpropanoic acid
(d) 3-Methylbutane carboxylic acid
Step by step video & image solution for what is the correct IUPAC name of the given compound? (a) 2,2-Dimethylbutanoic acid (b) 2-Carboxyl-2-methylbutane (c) 2- Ethyl-2-methylpropanoic acid (d) 3-Methylbutane carboxylic acid by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
A student named a certain compound as 2,3-diethylbutane. Its correct IUPAC name is
a) 2,3-dimethylhexane
b) 3,4-dimethylhexane
c) 2-ethyl-3-methylpentane
d) 2-ethylbutane
View SolutionWrite the structures of the following compounds and identify them as 1∘, 2∘ or 3∘ halides.
(a) 1-Bromo-2-methylpropane (b) 2-Chloro-2-methylpropane (c) 2-Bromo-3-methylbutane
(d) 3-Bromopentane (e) 2-Bromo-2-methylbutane (f) Neopentyl chloride.
View Solution
Knowledge Check
IUPAC name of carboxylic acid is
Aalkinoic acidBalkanoic acidCalkenoic acidDalkyl alkanoateWhat is the IUPAC name of the following compound ?
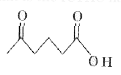 AHexa-2,6-dienone-6-olB2-Ketohexanoic acidC5-Ketohexanoic acidD2-Oxohexanoic acid
AHexa-2,6-dienone-6-olB2-Ketohexanoic acidC5-Ketohexanoic acidD2-Oxohexanoic acidWhich one of the following compounds gives carboxylic acid with HNO2?
A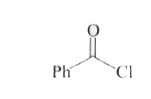 B
B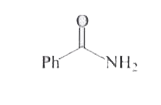 C
C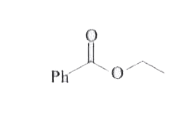 D
D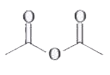
Write the structures of the following compounds and identify them as 1∘, 2∘ or 3∘ halides.
(a) 1-Bromo-2-methylpropane (b) 2-Chloro-2-methylpropane (c) 2-Bromo-3-methylbutane
(d) 3-Bromopentane (e) 2-Bromo-2-methylbutane (f) Neopentyl chloride.
View SolutionCH3−C∣CH3H−CHO+CH3MgBrEther−−→AH3O+−−−→B
The IUPAC name of B is :
(a) Pentan-2-ol " " (b) 2-Methylbutan-2-ol
(c) 2-Methylbutan-3-ol (d) 3-Methylbutan-2-ol
View SolutionWrite structures of the compounds whose IUPAC names are given below:
(a) 2-Methylbutan-2-ol (b) 1-Phenylpropan-2-ol
(c) 3, 5-Dimethylhexane-1,3,5-triol (d) Cyclohexylmethanol
View SolutionWhich one of the following compounds gives carboxylic acid with HNO2 ?
View SolutionWhich one of the following compounds gives carboxylic acid with HNO2?
View Solution
