Identify the solute and solvent in the option given below, for a solution as amalgam of mercury with sodium.
A
SoluteSolventSolidLiquid
B
SoluteSolventSolidSolid
C
SoluteSolventLiquidSolid
D
SoluteSolventSolidGas
Video Solution
Text Solution
The correct Answer is:C
SolutesolventCommon examplesLiquidSolidAmalgam of mercury with sodium
|
Similar Questions
Knowledge Check
In amalgam of mercury with sodium, solvent is
AmercuryBsodiumCamalgamDnone of theseIdentify the correct statement from the options given below for the galvanic cell :
ACathode in a voltaic cell loose weight during reactionBΔG is positive for spontaneous cell reaction in voltaic cellCThe voltaic cell is working in reverse direction if the e.m.f of a cell is negativeDThe cell reaction is spontaneous in galvanic cell if Ecell is less than external potential applied in cellWhen a solute is dissolved in a solvent
Avapoure pressure of the solvent is increasedBfreezing point of the solution becomes more than that of solventCboiling point of the solution becomes lower thant that of solventDvapour pressure of the solvent is decreased
Identify the correct option regarding the structure given below .
View Solution(a) Identify the solute and solvent in tincture of iodine.
(b) Why is Tyndall effect not seen in a true solution ?
View SolutionSolvent is a substance which is dissolved in solute to form a solution.
View SolutionDistinguish between a solute and a solvent
View SolutionIdentify A,B, and C in the given below blood cloting process .
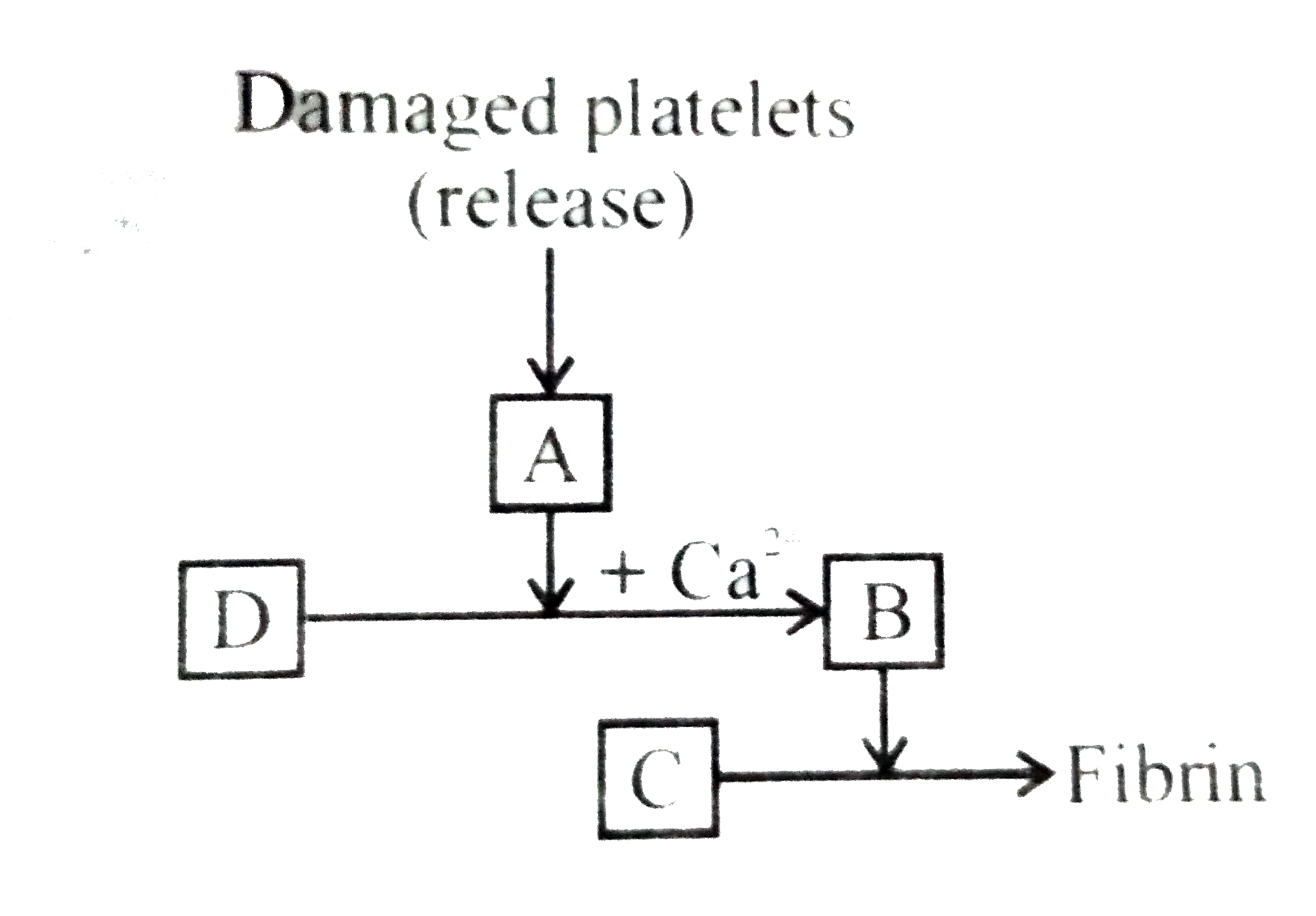
Options :−
View Solution
