Decomposition of ammonium nitrite is an examle of
A
bimolecular reaction
B
unimolecular reaction
C
Both (a) and (b)
D
None of the above
Video Solution
Text Solution
The correct Answer is:B
Decomposition of ammonium nitrite is an example of unimolecular reaction.
|
Similar Questions
Knowledge Check
The following data are for the decomposition of ammonium nitrite in aqueous solution.:
Vol ofN2(cm3)6.259.011.4213.6535.2Time (min)10152025∞
The order of reaction is :A3B2C1DzeroThe following data are for the decomposition of ammonium nitrate in aqueous solution :
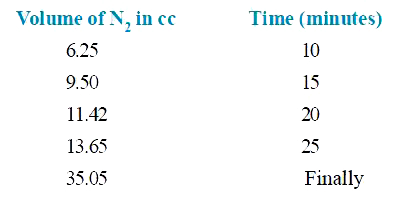
The order of the reaction is:AZeroBOneCTwoDThreeFerrocene is an examle of
Asandwiched comlexBpi bonded complexCa complexin which all the five carbon atoms of cyclopentadiene anion are bonded to the metalDAlll of the above
