C4H10O gives white precipitate immediately with conc. HCl in the presence of anhydrous ZnCl2. Alcohol can be
A
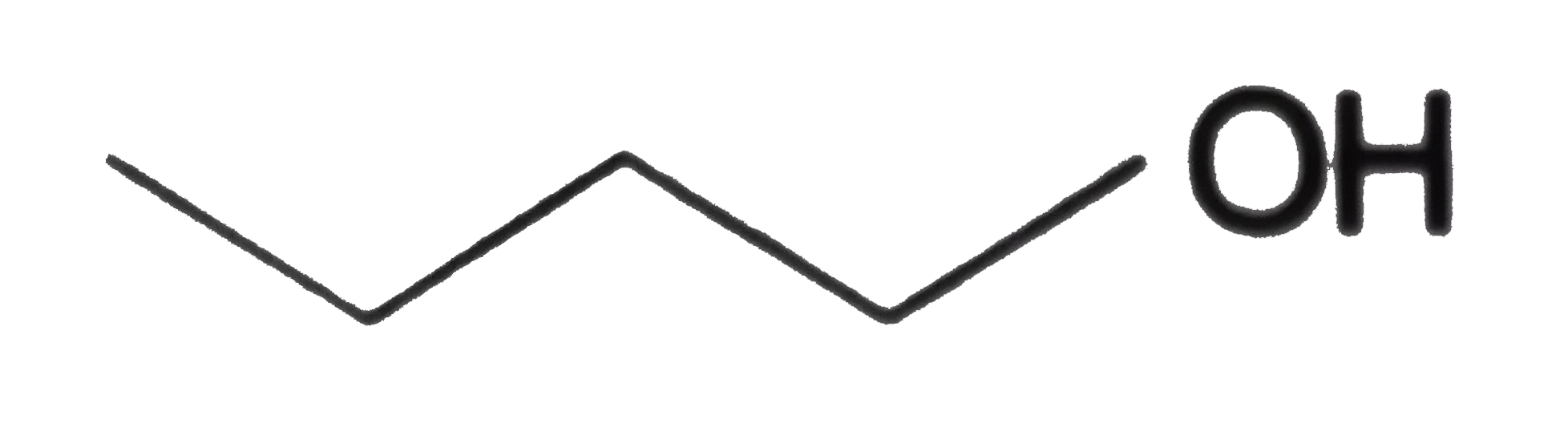
B
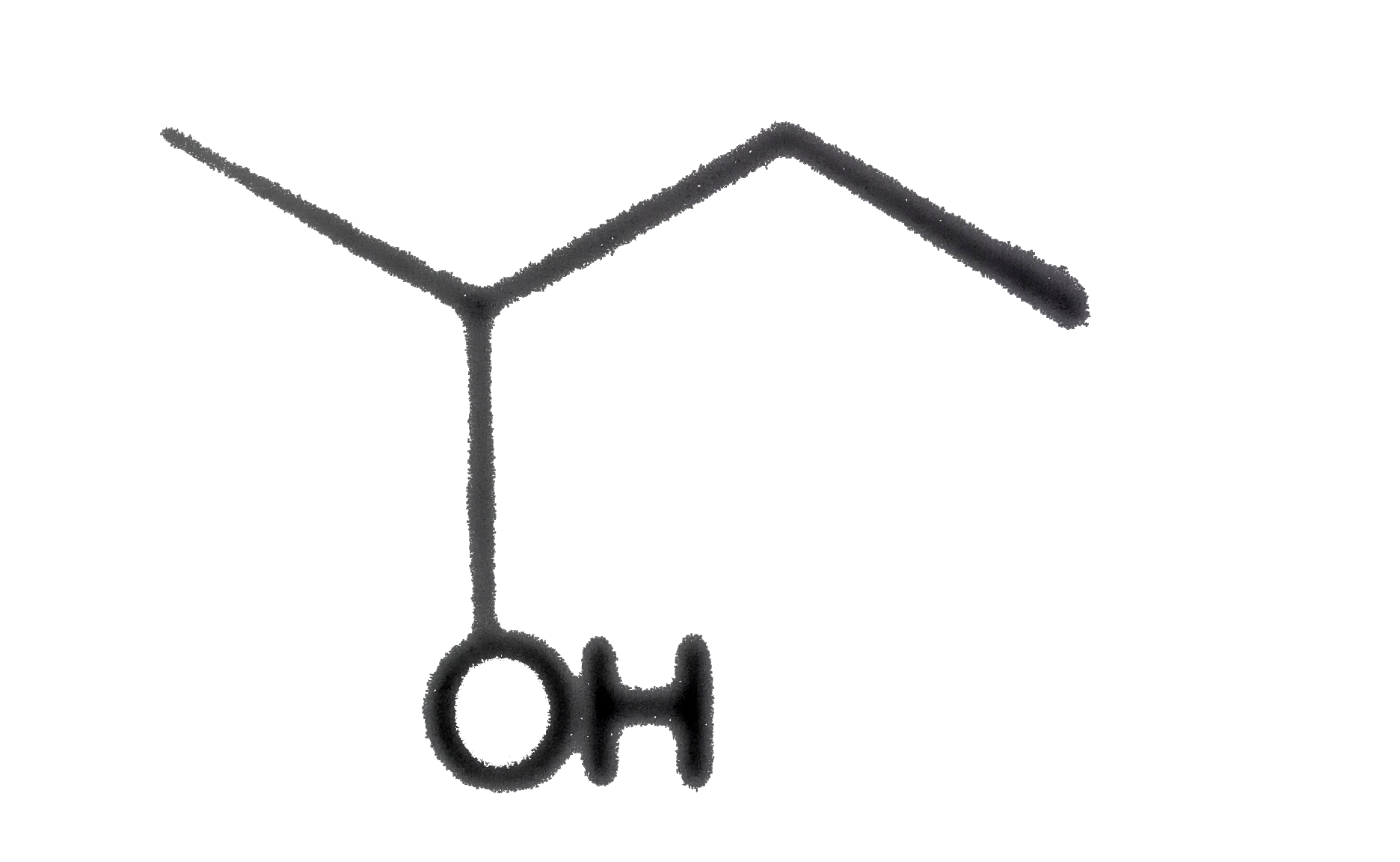
C
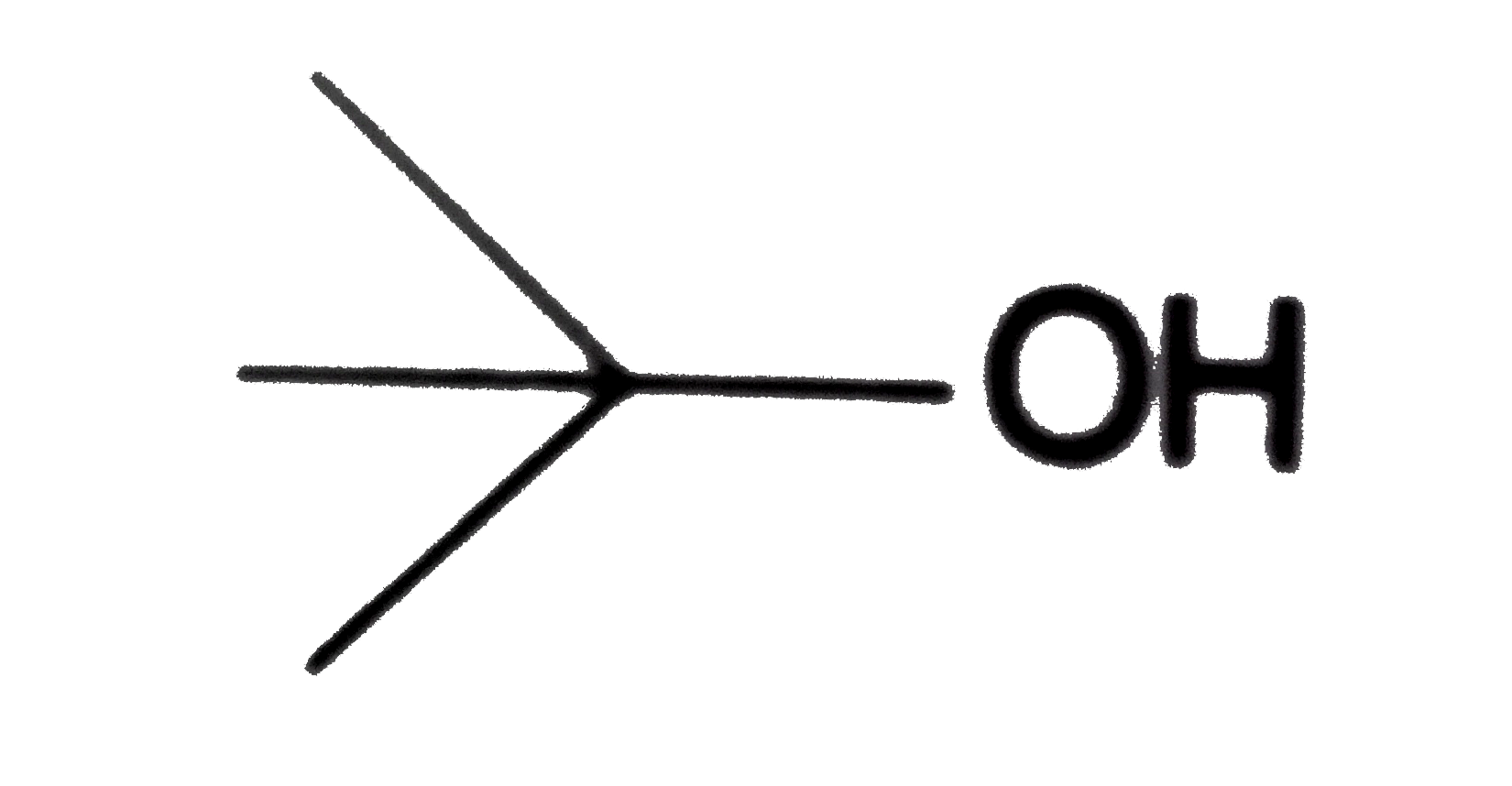
D
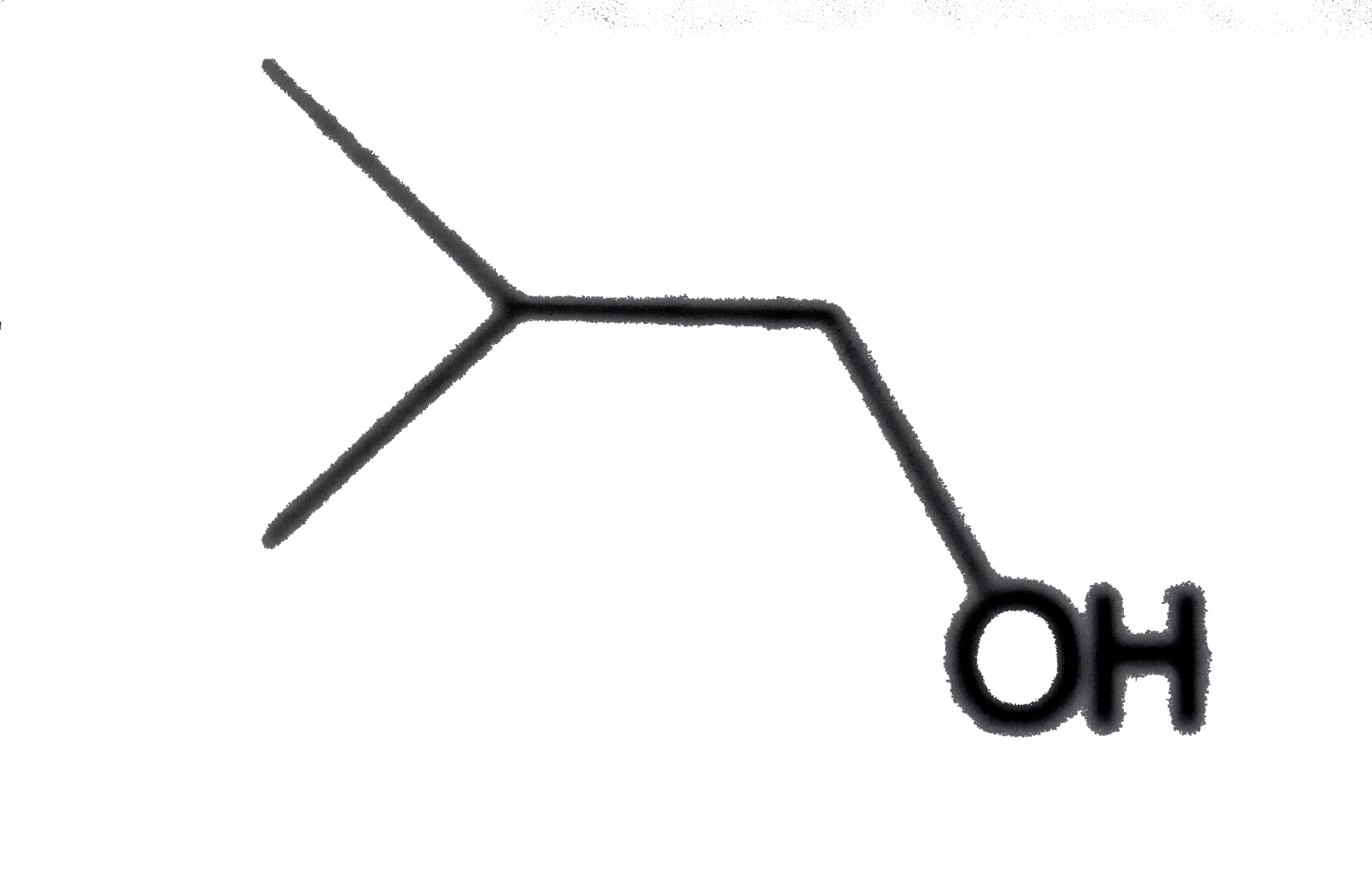
Text Solution
The correct Answer is:C
3∘ alcohol reacts most readily with conc. HCl forming white precipitate.
R3COHTertiaryalcoholConc. HCl−−−−−−−→Anhy. ZnCl2R3CClTurbidity appearsimmediately+H2O
|
Similar Questions
Knowledge Check
Which alcohol is most reactive towards HCl in the presence of anhydrous ZnCl2?
AprimaryBsecondaryCtertiaryDall of theseEther on heating with acetyl chloride in presence of anhydrous ZnCl2gives :
Aethyl alcoholBethyl chlorideCethyl acetateDethyl chloride and ethyl acetateWhen phenol Is heated with ammonia at 300∘C in the presence of anhydrous ZnCl2, it is converted into
AanilineBCyclohexanolCcyclohexlamineDp−phenuylendiamine
Of the two alcohols, (a) CH2=CH−CH2OH and (b) CH2=CH−CH2−CH2OH, which one will react more easily with conc. HCl in the presence of ZnCl2?
View SolutionThe hydroxyl compound that gives a precipitate immediately when treated with concentrated HCl and anhydrous ZnCl2 is :
View SolutionWhen alcohol are reacted by HCl,in presence of anhydrous ZnCl2, the later one behaves as a
View SolutionWhich of the following alcohols is most reactive with HCl in the presence of ZnCl2?
View SolutionWhich of the following is the most reactive with HCl in the presence of ZnCl2?
View Solution

