A simple method to remove peroxides from ethers is to treat them with an aqueous solution of :
A
KI
B
KCNS
C
Na2S2O3
D
Br2
Video Solution
Text Solution
The correct Answer is:A
Ether peroxide oxidises KI into I2 and itself gets reduced to ether. Therefore, KI is added to remove peroxides from ethers.
2I−→I2+2e−
Ether peroxide +2e−→Ether +O2
|
Similar Questions
Knowledge Check
A simple method to remove peroxides from ehters is to treat them with an aqueous solution of
AFeSO4BKCNSCNa2S2O3DBr2A simple mehtod to remove peroxide form ehters is to treat them with an aqueous solution
AKIBKCNSCNa2S2O3DBr2Which product is formed when the compound is treated with concentrated aqueous KOH solution ?
A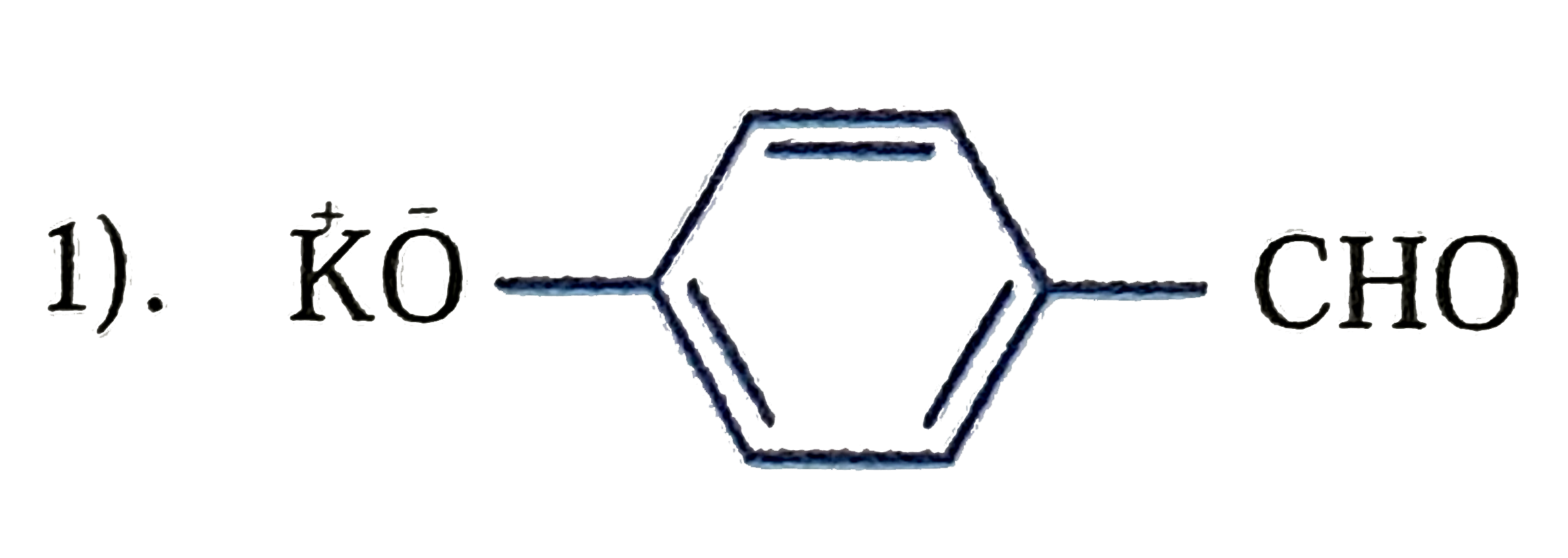 B
B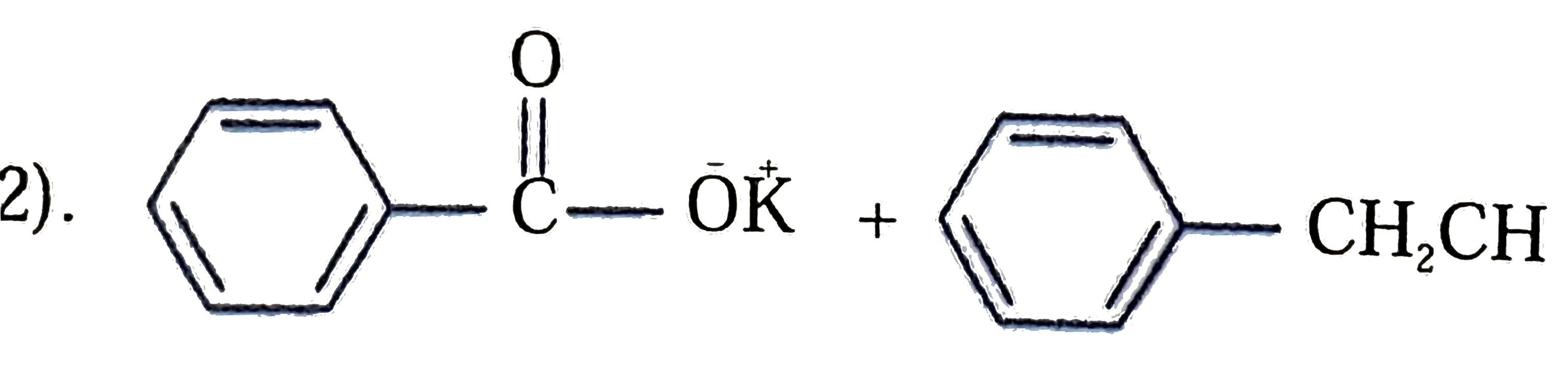 C
C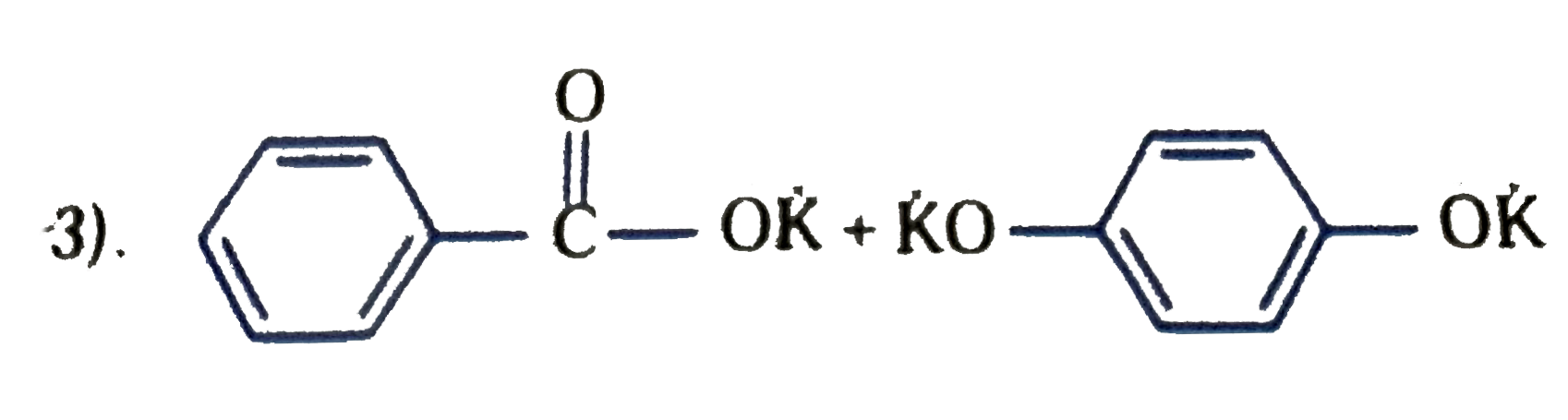 D
D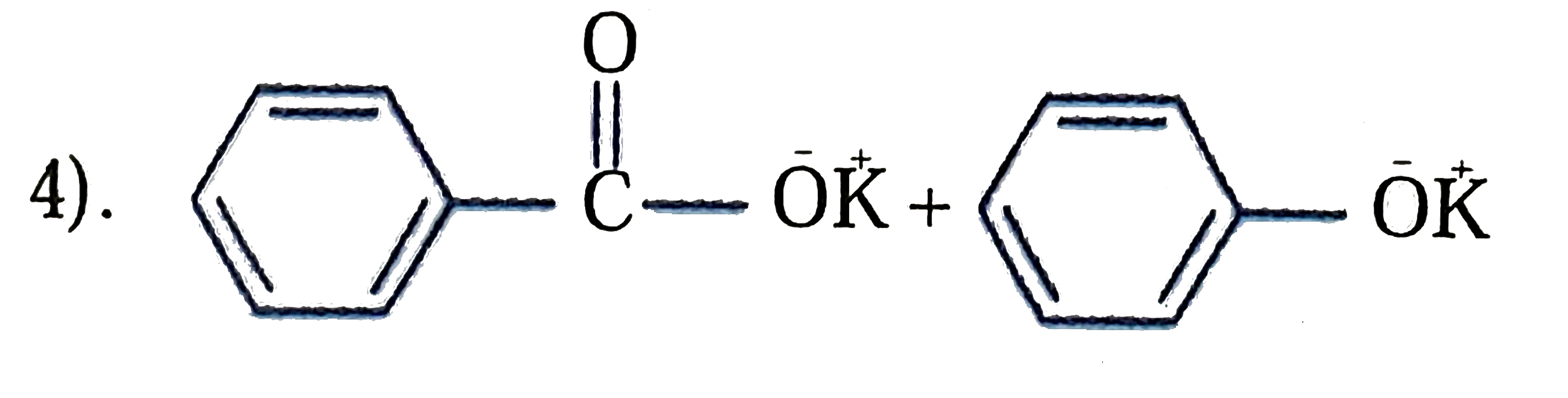
In Dumas' method , N2 gas obtained from the organic compounds is collected over an aqueous solution of
View SolutionWhich product is formed when the compound
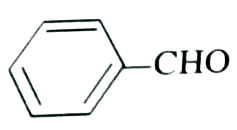 is treated with concentrated aqueous KOH solution?
is treated with concentrated aqueous KOH solution?
View SolutionWhich product is formed when the compound

is treated with concentrated aqueous KOH solution
View SolutionPresence of peroxide in ethers of old stock can be tested by first treating them with FeSO4 solution and then adding an aqueous solution of ………&from red colour solution
View SolutionAqueous solution of hydrogen peroxide .
View Solution

