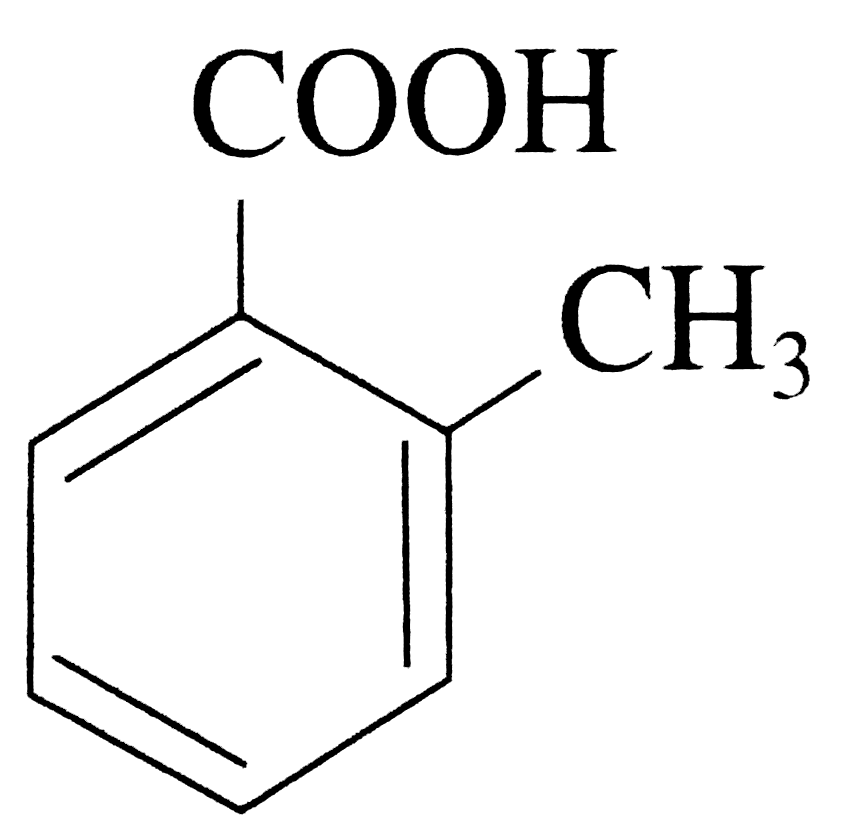
The strongest acid among the following aromatic compound is
A
o-nitrophenol
B
phenol
C
p-nitrophenol
D
m-nitrophenol
Video Solution
Text Solution
The correct Answer is:B
p-nitrophenol is more acidic because pressence of electron withdrawing group at o- or p-positions increases the acidic strength.
|
Similar Questions
Knowledge Check
The strongest acid among the following aromatic compounds is
Aortho-nitrophenolBpara-chlorophenolCpara-nitrophenolDmata-nitrophenolThe strongest acid among the following is
Ap−Cl−C6H4COOHBp−OHC6H4COOHCC6H5COOHDp−NO2C6H4COOHThe strongest acid among the following is
A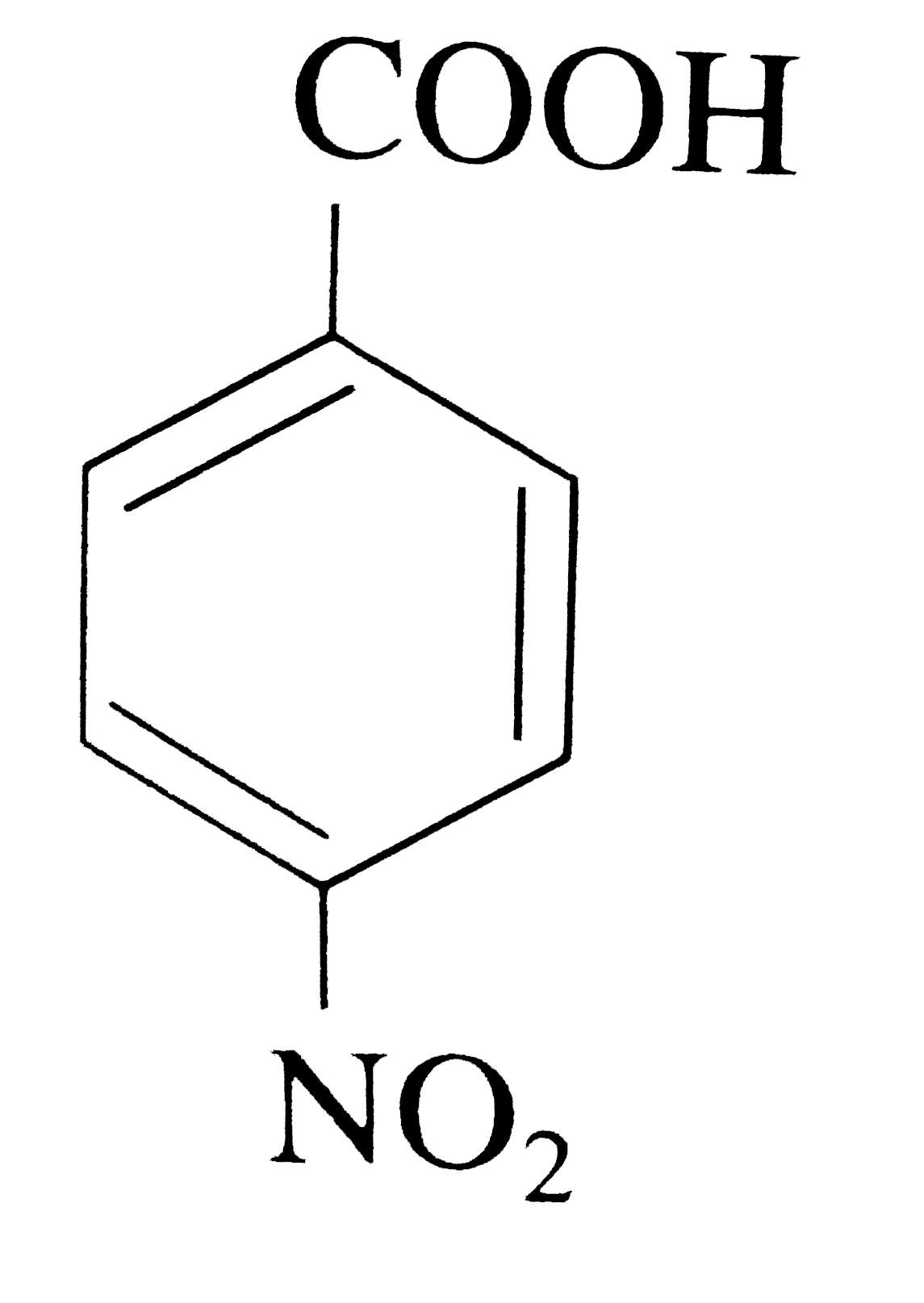 B
B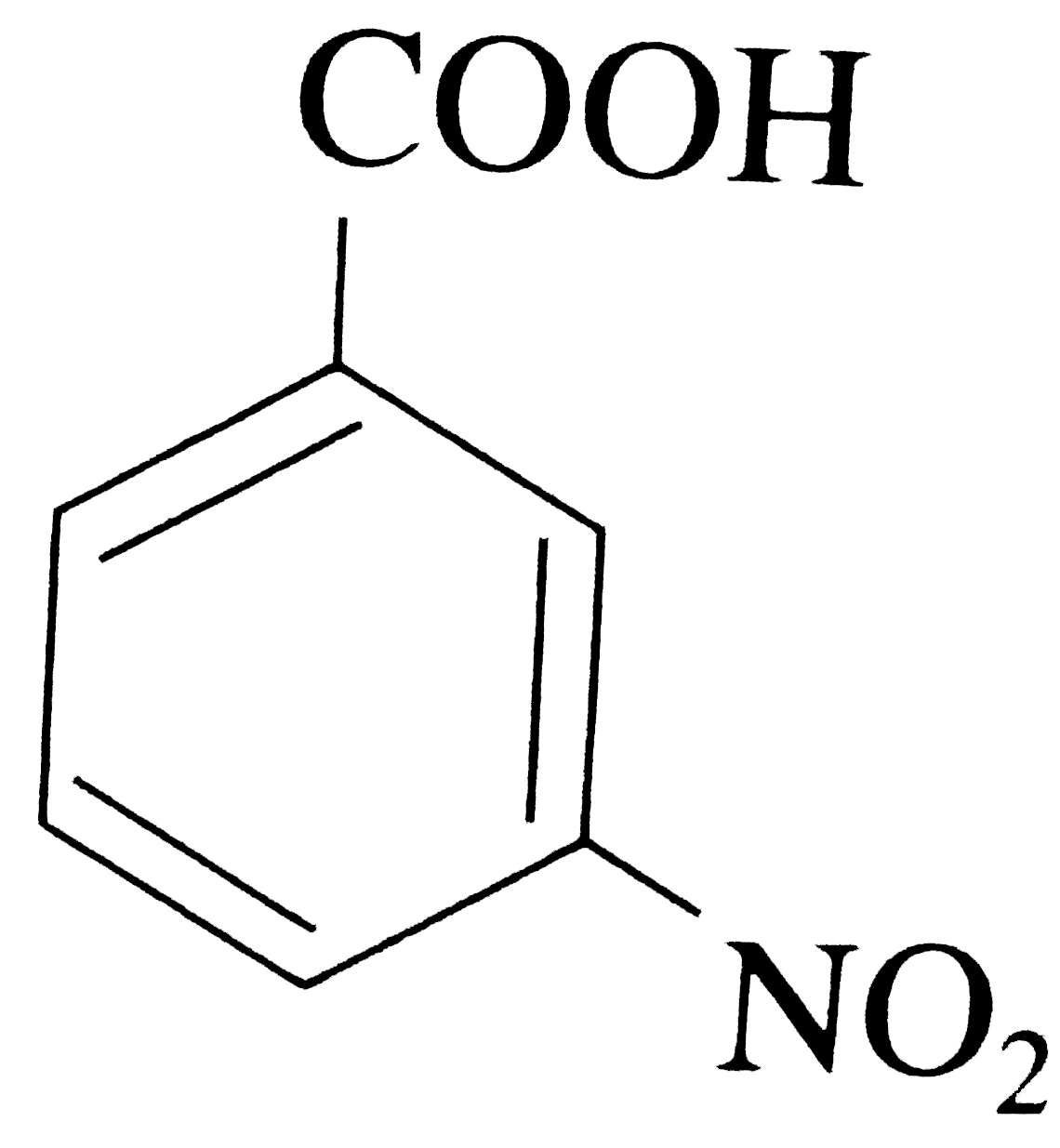 C
C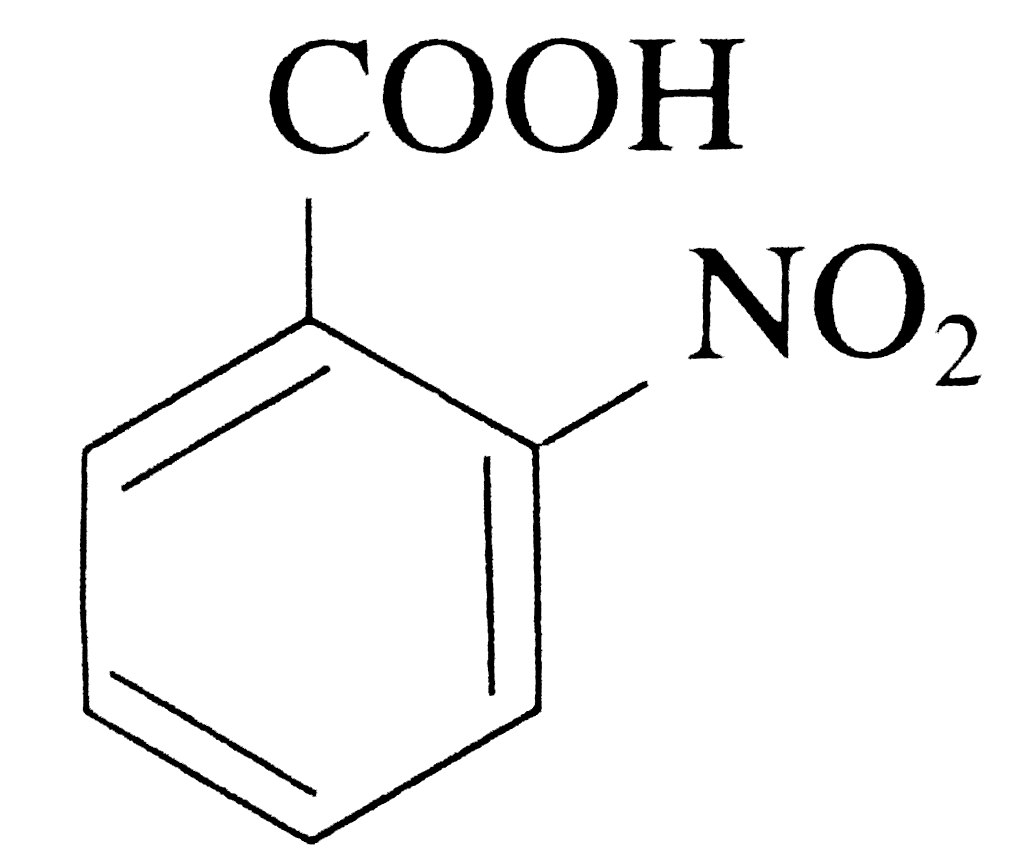 D
D