Discuss the effect of catalyst on the kinetics of a chemical reaction with a suitable diagram. Solution in Telugu
Video Solution
Text Solution
The substance which increases the rate of reaction without being
consumed in the chemical reaction is called a catalyst.
In presence of catalyst the reaction proceeds in a new path which lowers the activation energy.
Eg:N2(g)+3H2(g)Fe(s)⇐=⇒2NH3(g)
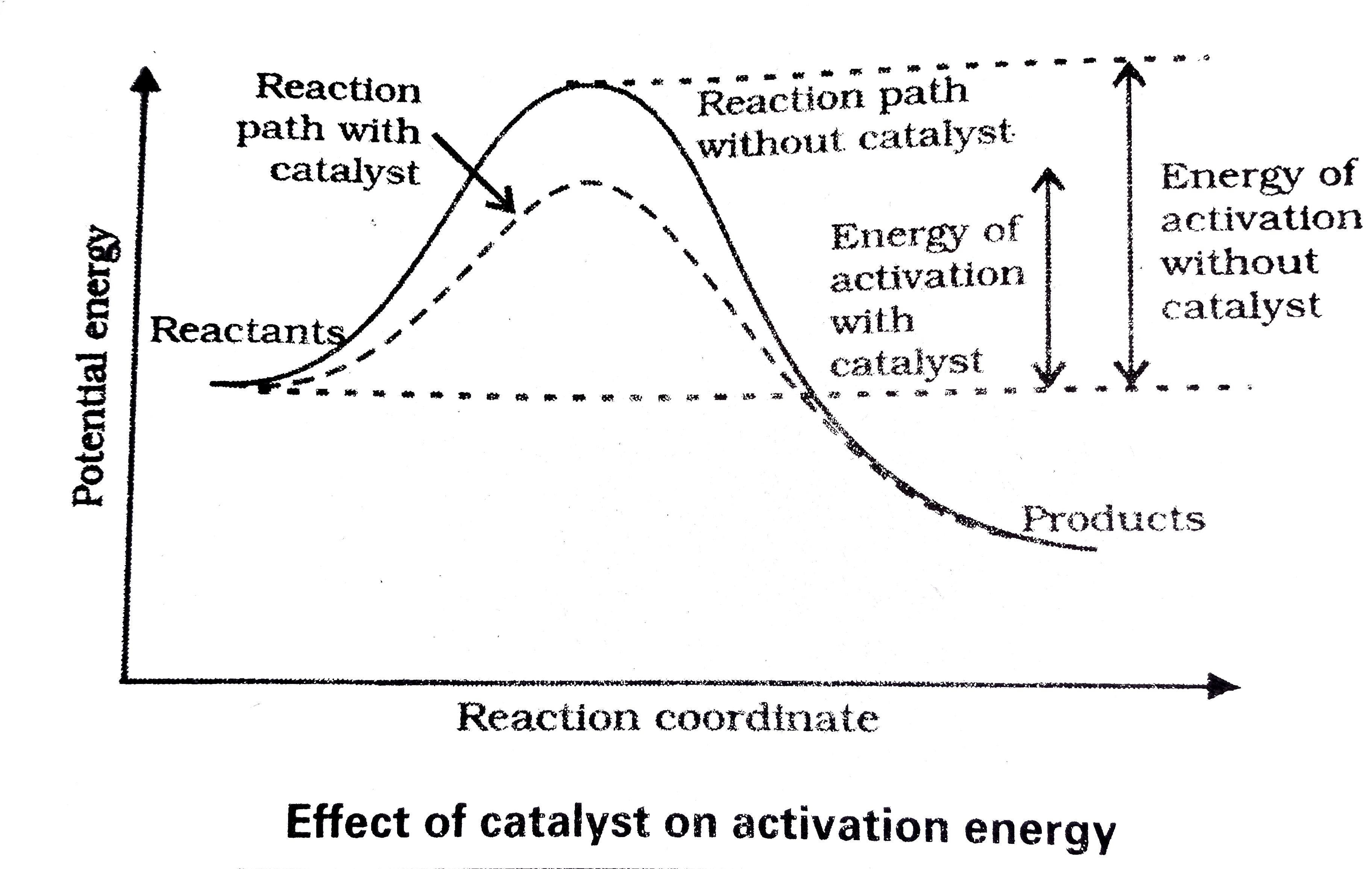
|
Similar Questions
How does a catalyst work in a chemical reaction? Discuss the catalysis with suitable examples.
View Solutionकिसी रासायनिक अभिक्रिया में उत्प्रेरक का प्रभाव परिवर्तित करना होता है
View SolutionExplain the effect of catalyst on the rate of reaction with diagram.
View Solutionरासायनिक अभिक्रिया में उत्प्रेरक
View SolutionDiscuss the effect of catalyst on the activation energy.
View SolutionExplain the effect of catalyst on the rate of reaction with diagram.
View SolutionDiscuss the effect of catalyst on the activation energy.
View Solutionenergy diagram #!#effect of catalyst
View Solution
