How would you determine the standard electrode potential of the system Mg2+/Mg? Solution in Telugu
Video Solution
Text Solution
E0 vlaue of Mg2+/Mg electrode is determined by setting up an electrochemical cell. For this purpose, a Mg electrode is dipped in 1MMgSO4 solution, which acts as one half cell i.e., oxidation half-cell.
In the same way, the standard hydrogen electrode acts as the other half-cell i.e., reduction half cell. The deflection of voltmeter placed in the cell circuit is towards the Mg electrode which indicates the direction of flow of current.
The cell may be represented as : Mg∣∣Mg2+(1M)∣∣∣∣H+(M)∣∣H2(1atm). Pt The reading as given by boltmeter gives E0cell
E0cell=E0(H+/1/2H2)−E0(Mg2+/Mg)=0−E0(Mg2+/Mg)
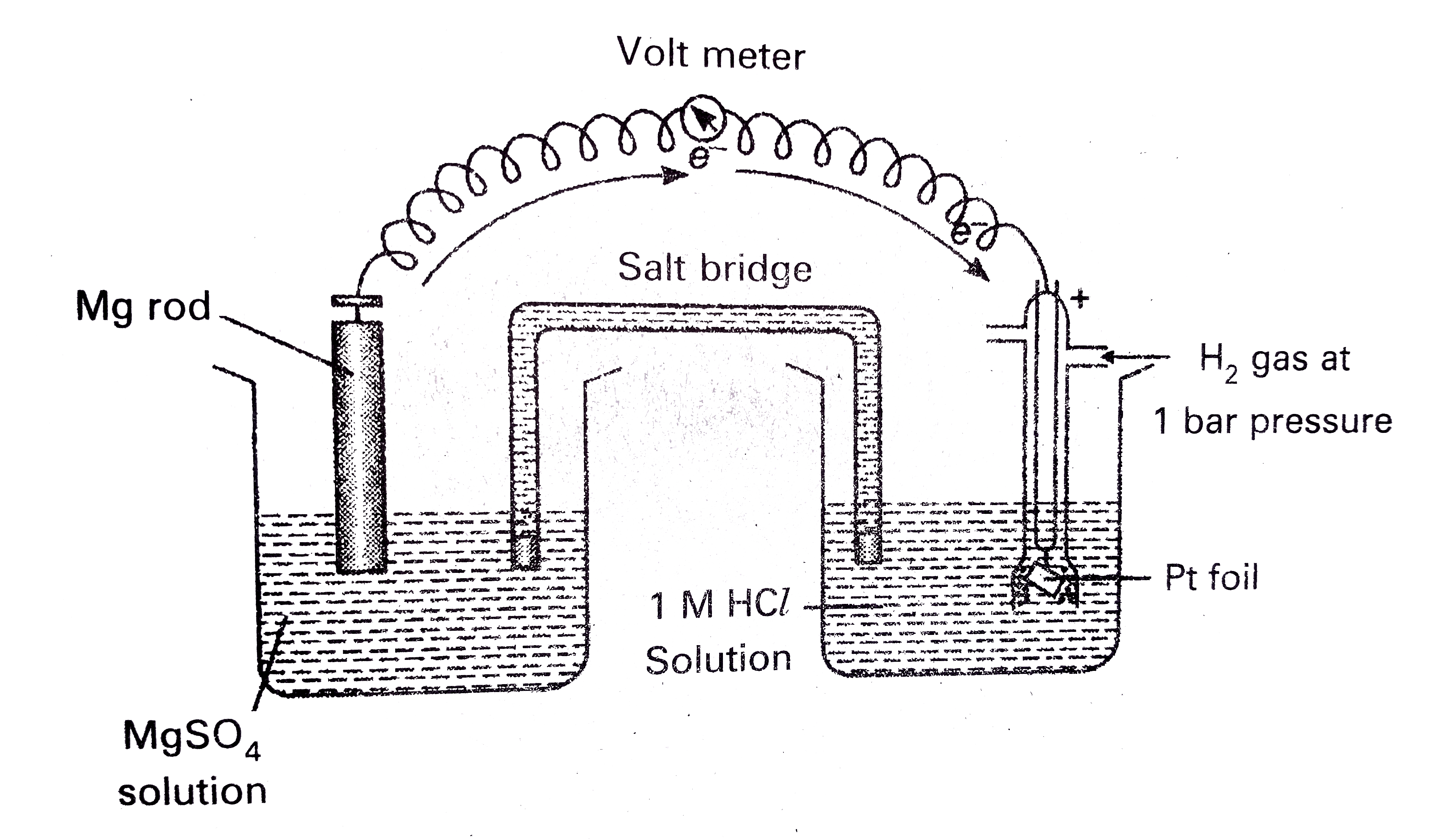
|
Similar Questions
Mg2+/Mg इलेक्ट्रोड विभव क्या है जबकि उसे जो विलयन में डुबोया जाता है उसमें Mg2+ की सान्द्रता (0.01 M है? E0Mg2+/Mg=−2.36V.
View SolutionHow would you determine the standard electrode potential of the system Mg^(2+) Mg ?
View SolutionHow would you determine the standard reduction potential of the system Mg2+∣Mg?
View SolutionHow would you determine the standard electrode potential of the system Mg2+∣Mg ?
View SolutionHow would you determine the standard electrode potential of the system Mg^(2+) //Mg ?
View SolutionHow would you determine the standard electrode potential of the system Mg^(2+)//Mg ?
View SolutionHow would you determine the standard electrode potential of the system Mg2+/Mg?
View SolutionHow would you determine the standard electrode potential of the system (Mg^(2+) )/ Mg ?
View Solution
