Action of soap is due to emulsification and micelle formation. Comment.Solution in Telugu
Soap is sodium stearate, C17H35COONa in water soap gives the ions stearate anion and sodium ion.
C17H35COONa→C17H35COO−Stearate ion+Na+
The anion of soap =C17H35COO−
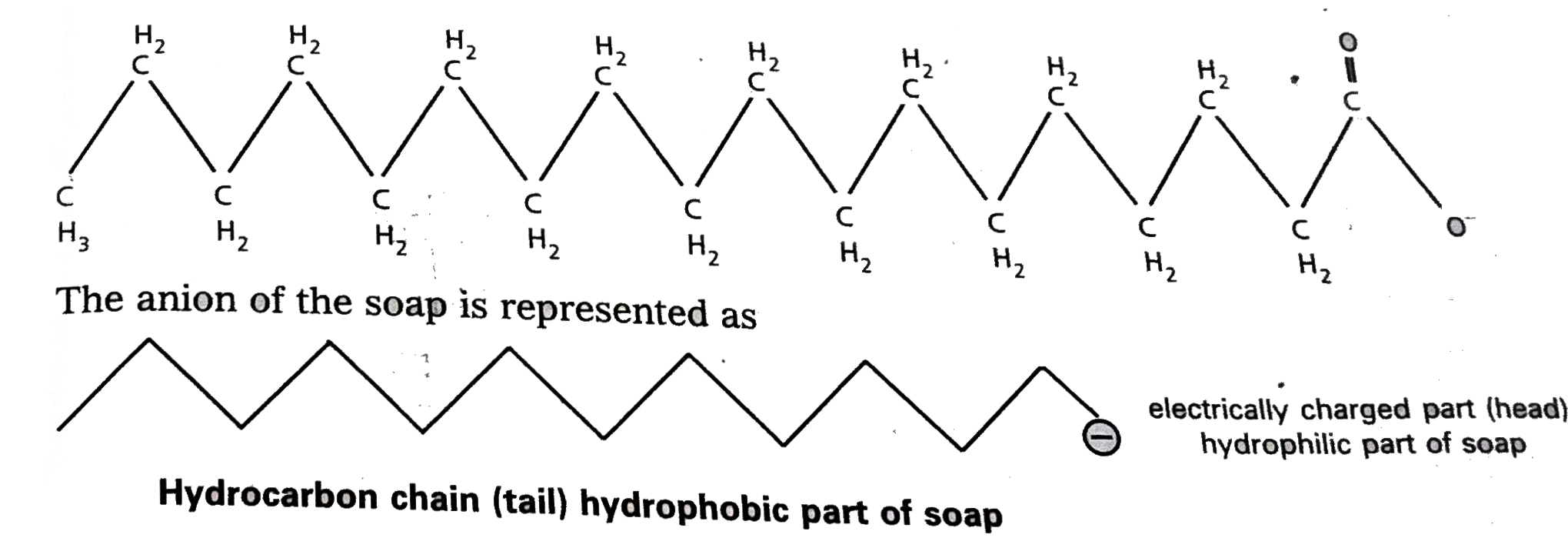
Cleaning action of soap : Soap anions form a micelle. The grease or dirt of the cloth are absorbed into the interior of the micelle. The tails of the anion are pegged into micelle and these micelle are washed away with the soap solution. Soap thus, functions as an emulsifying agent for the water dirt emulsion. The emulsified grease or dirt is then washed away with soap solution

Similar Questions
साबुन की क्रिया पायसीकरण एवं मिसेल बनने के कारण होती है, इस पर टिप्पणी कीजिए।
View SolutionA micelle formed during the cleansing action by soap is
View SolutionA micelle formed during the cleansing action by soap is
View SolutionMicelles are formed from concentrated soap solutions above critical micelle concentration. Micelles are regarded as associate colloids as soap ions associate to give micelles.
During micelle formation ........... in solution
View SolutionA micelle formed during the cleansing action by soap is
View SolutionWhich of the following options are correct? (a)Micelle formation by soap in aqueous solution is possible at all temperatures (b)Micelle formation by soap in aqueous solution occurs above a particular concentration (c)On dilution of soap solution micelles may revert to individual ions (d)Soap solution behaves as a normal strong electrolyte at all concentrations
View SolutionDiscuss cleansing action of soap by micelle formation.
View SolutionExplain micelle formation in soap solution.
View Solution
