How is ozone prepared from oxygen ? Explain its reaction with
C2H4Solution in Telugu
Preparation of Ozone :
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%) The product obtained is known as ozonised oxygen.
3O2→2O3ΔH∘=142kJ/mole
→ The formation of ozone is an endothermic reaction.
→ It is necessary to use silent electric discharge in the preparation of O3 to prevent its de composition
Reaction with C2H4 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
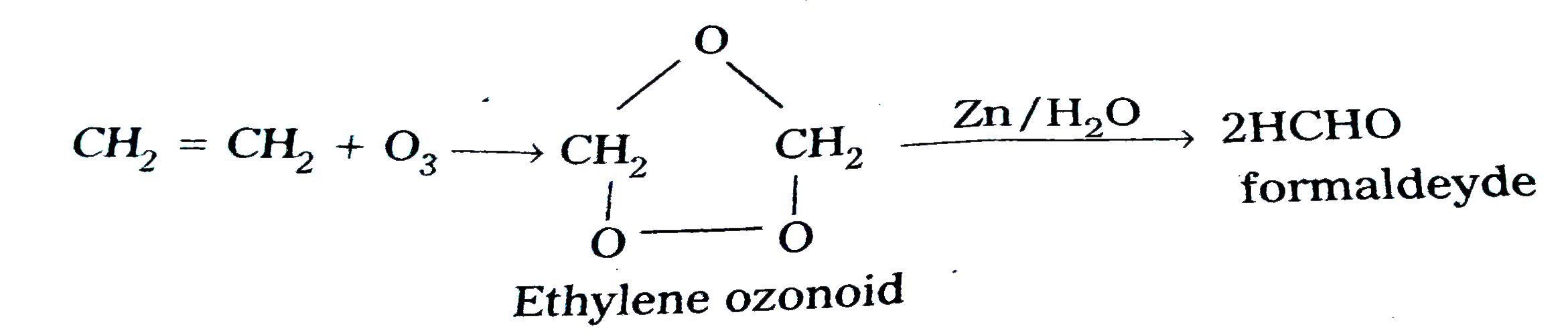
Similar Questions
How will you isolate CH4,C2H4andC2H2 from their mixtures ?
View SolutionHow will you prepare ozone by laboratory method? Explain the structure of ozone.
View Solution15% of oxygen is converted to ozone. The mass of ozone that can be prepared from 67.2 L of oxygen at N.T.P. will be
View SolutionAssertion (A) : Ozone is prepared from oxygen in a silent electrical dicharge.
Reason (R) : Formation of ozone from oxygen is an endothermic process.
View SolutionWhich of the following is most reactive in its reaction with C2H4?
View SolutionOzone is prepared by passing silent electric discharge through oxygen. In this reaction.
View SolutionOzone is prepared by passing silent electric discharge through oxygen. In this reaction
View SolutionHow is ethylamine prepared from C_(2)H_(5)NO_(2) and CH_(3)CH_(2)CONH_(2) ? Mention its reaction with CH_(3)COCl and C_(2)H_(5)Cl
View Solution
