Compared to the changes in atomic and ionic sizes of elements of 3d and 4d series, the : change in radii of elements of 4d and 5d series is virtually the same Comment.Solution in Telugu
Compared to the change in atomic and ionic sizes of elements 3d and 4d series, the change in radii of elements 4d and 5d series is virtually the same. This is due to the intervention of the 4f orbitals which must be filled before the 5d series of elements begin. The filling of 4f before 5d orbitals results in a regular dectease in atomic radii called lanthanoid contraction. The consequence of lanthanoid contraction is that the 4d and 5d series exhibit same size of radii.
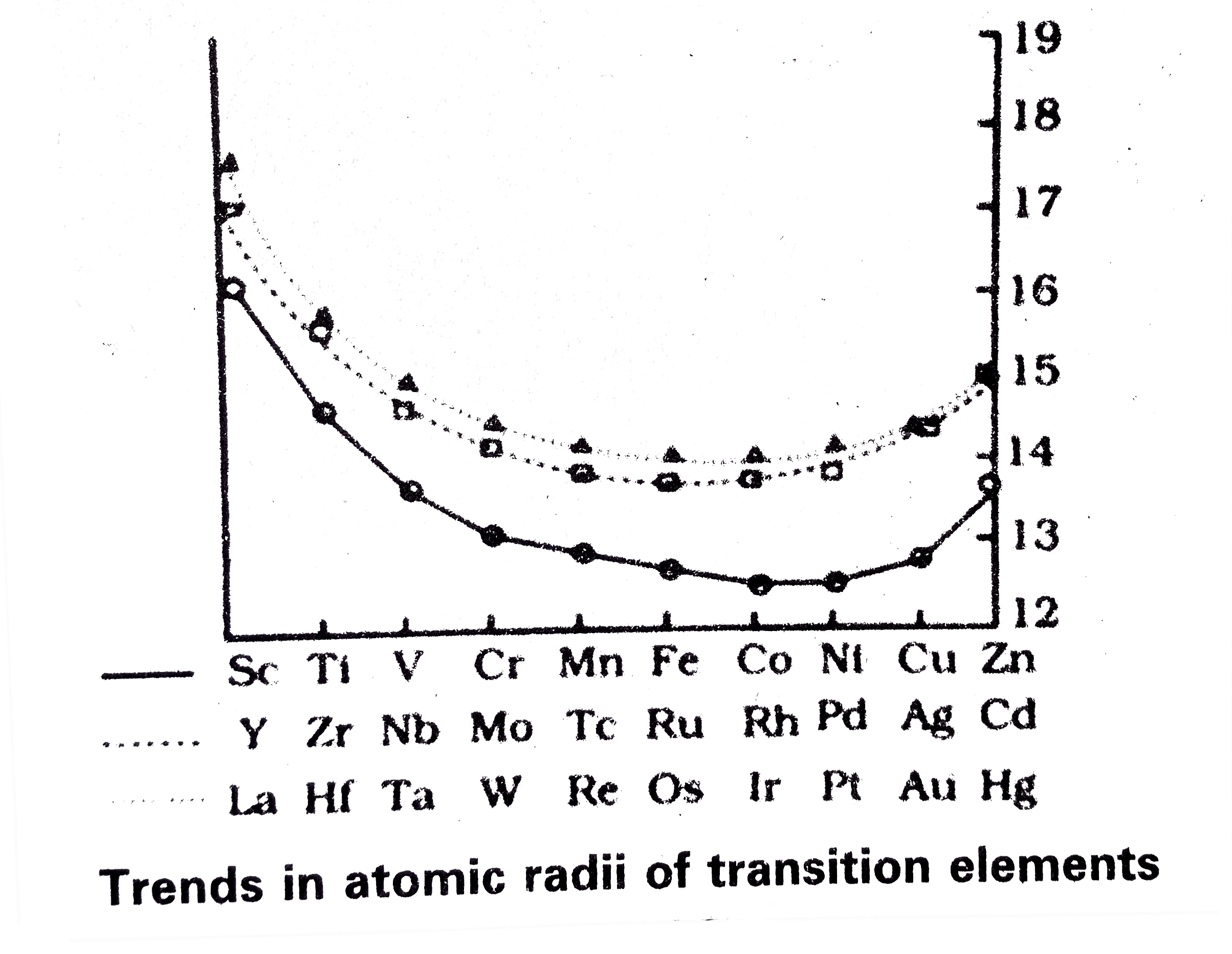
Similar Questions
Zr is in 4d-series, Hf is in 5d-series. But their atomic radii is same. Why?
View SolutionZr is in 4d-series, Hf is in 5d-series. But their atomic radii is same. Why?
View SolutionThe first ionization enthalpy of 5d series elements are highest as compared to 3d and 4d elements because
View SolutionAssertion : In transition elements radii of 5d series are virtually the same as those of the corresponding members of 4d series. Reason : The filling of 4f orbitals before 5d orbitals results in regular decrease in atomic radii.
View Solution(A) Metals of 4d and 5d series have greater enthalpies of atomization than the corres- ponding 3d series. (R) 4d and 5d series elements are smaller than 3d series
View SolutionAccount for the following trends in atomic and ionic radii of transition metals. The atomic radii of elements in 4d series are more than that of corresponding elements in 3d series.
View SolutionAccount for the following trends in atomic and ionic radii of transition metals. The atomic radii of the corresponding elements in '4d' series and 5d series are virtually the same.
View Solution3d-, 4d- and 5d- series consists of 10 elements each? Explain.
View Solution

