Discuss the nature of bonding and magnetic behaviour in the [Co(C2O4)3]3− Co-ordination entities on the basis of valence bond theory.Solution in Telugu
[Co(C2O4)3]3− : In this complex, the oxidation state of Co is + 3.
Outer configuration of Co=3d74s2
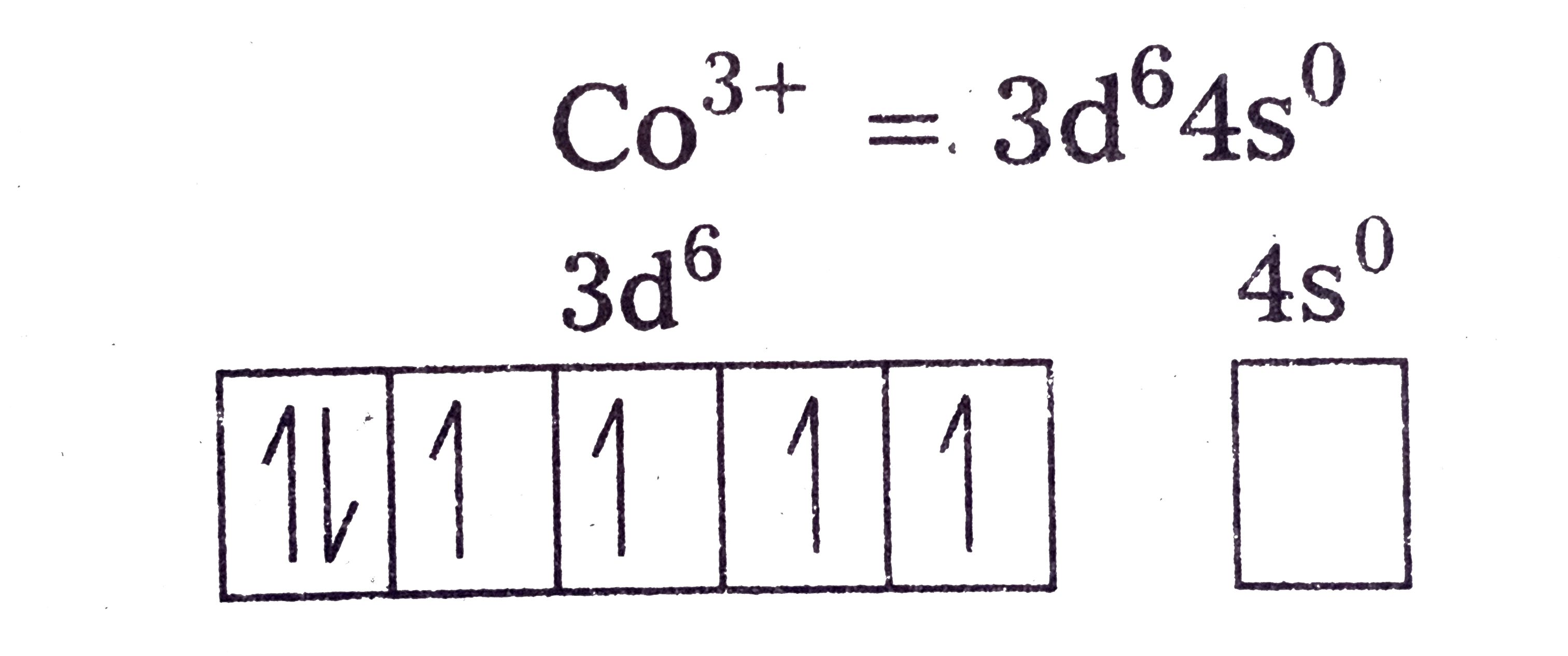
Oxalate ion being a strong field ligand pair .up the 3d electrons, thus two out of the five 3d-orbitals are available for oxalate ions.
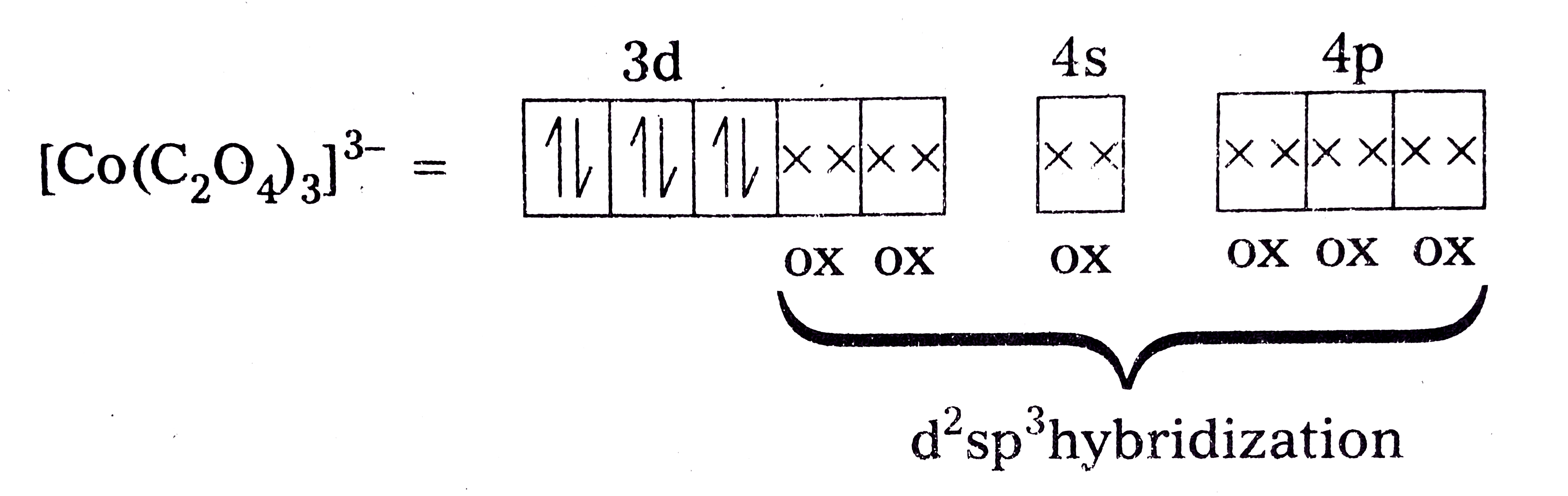
Since, all the electrons are paired, this complex is diamagnetic. It is an inner orbital complex because of the involvement of (n - 1) d-orbital for bonding,
Similar Questions
Discuss the nature of bonding in the following coordination entities on the basis of Valence Bond Theory :
[FeF6]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of Valence Bond Theory :
[Co(C2O4)3)]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of Valence Bond Theory :
[CoF6]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of valence bond theory: [FeF6]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of valence bond theory: [Co(C2O4)3]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of valence bond theory: [CoF6]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
[FeF6]3−
View SolutionDiscuss the nature of bonding in the following coordination entities on the basis of valence bond theory.
[Co(C2O4)3]3−
View Solution
