[NiCl4]2− is paramagnetic while [Ni(CO)4] is diamgnetic though both are tetrahedral. Why ?Solution in Telugu
In the complex [NiCl4]2−, Ni is in +2 oxidation state and has the configuration 3d84s0 . The Cl− ion being a weak field ligand cannot pair the two unpaired electrons present in 3d-orbitals. This means that 3d-orbitals are not involved in hybridization. Thus, the complex is sp3 hybridized (tetraheqral) and is paramagnetic in nature.
Outer configuration of Ni atom =
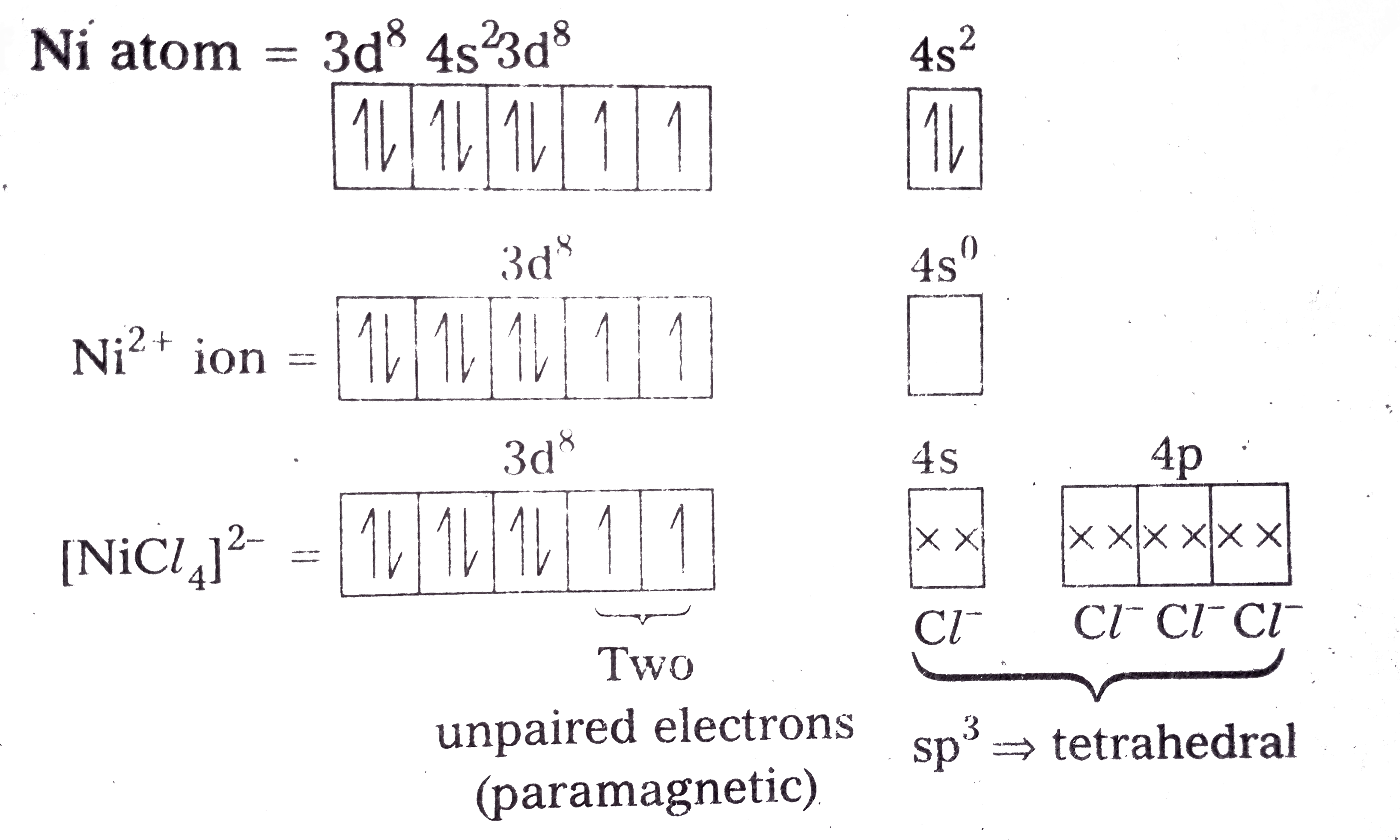
In the complex [Ni(CO)4], the oxidation state of nickel is zero and electronic configuration is 3d84s2. In the presence of the ligand co, the 4s-electrons shift to the two half-filled 3d-orbitals and make all the electrons paired. The valence 4s and 4p-orbitals are involved in hybridization. Thus, the complex is tetrahedral but diamagnetic in nature.
Outer configuration of Ni atom = 3d84s2
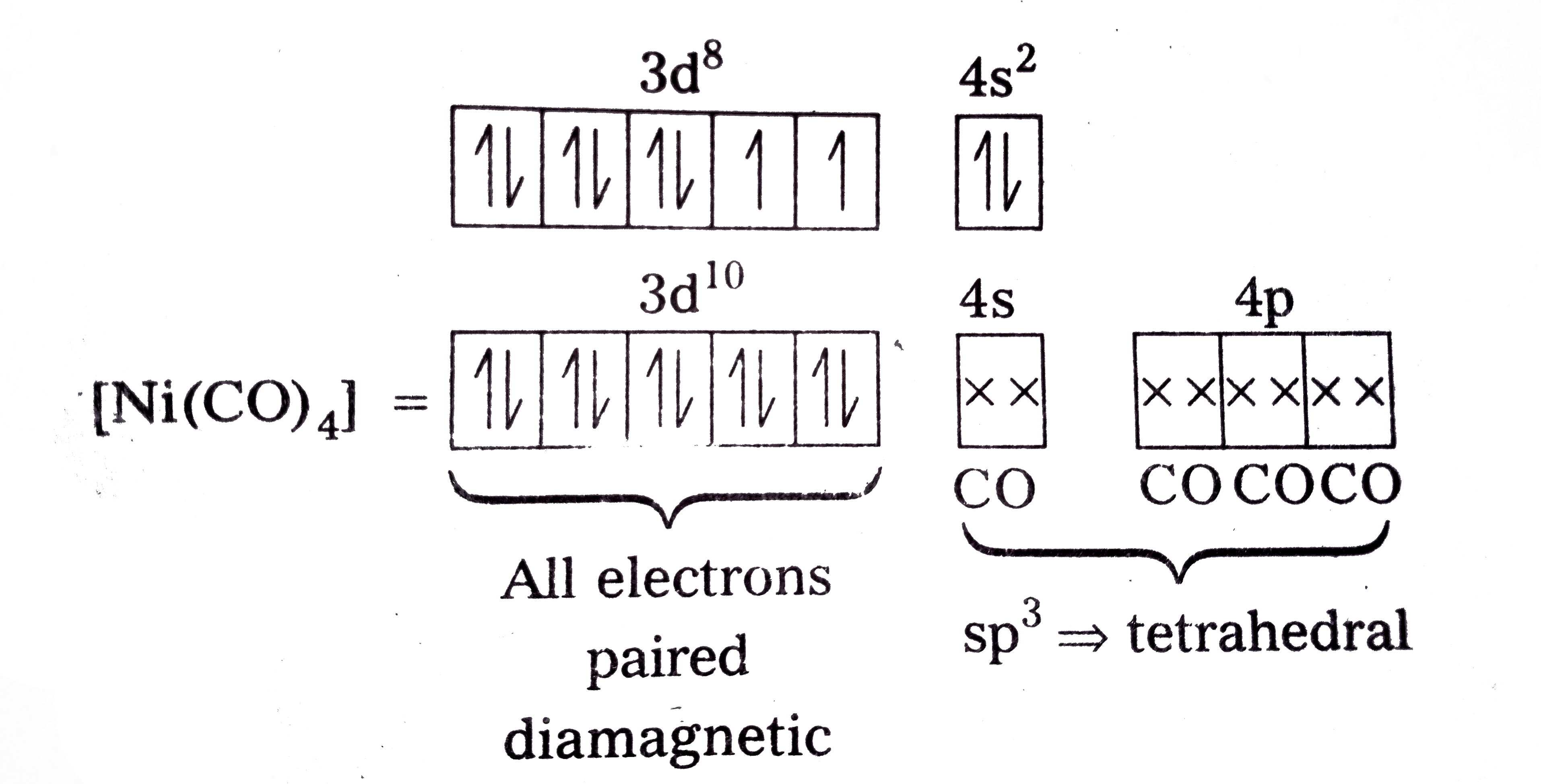
Similar Questions
[NiCl_(4)]^(2-) is paramagnetic while [Ni(CO)_(4)] is diamagnetic though both are tetrahedral. Why ?
View Solution[NiCl4]2− अनुचुंबकिय है जबकि [Ni(CO)4] प्रतिचुंबकिय है, जबकि दोनों चतुष्फलकीय है, क्यों ?
View Solution[NiCl_(4)]^(2-) is paramagnetic while [Ni(CO)_(4)] is diamagnetic though both are tetrahedral. Why?
View Solution[NiCl4]2− अनुचुंबकिय है जबकि [Ni(CO)4] प्रतिचुंबकिय है यधपि दोनों चतुष्फलकीय है, क्यों?
View SolutionNicl4]2− is paramagnetic while [Ni(CO4)] is diamagnetic though both are tetrahedral. Why
View Solution[NiC4]2−It is paramagnetic while Ni[CO4] is diamagnetic though both are tetrahedral Why
View Solution[NiCl_(4)]^(-2) is paramagnetic while [Ni(CO)_(4)] is diamagnetic though both are tetrahedral. Why?
View Solution[NiCl_(4)]^(2-) is paramagnetic while [Ni(CO)_(4)] is diamagnetic though both are tetrahedral. Why ?
View Solution
