Although chlorine is an electron withdrawing group , yet it is ortho-, para-directing in electrophilic aromatic substitution reactions . Why ? Solution in Telugu
Chlorine withdraws electrons through inductive effect and releases electrons through resonance . Through inductive effect , chlorine destabilises the intermediate carbocation formed during the electrophilic substitution .
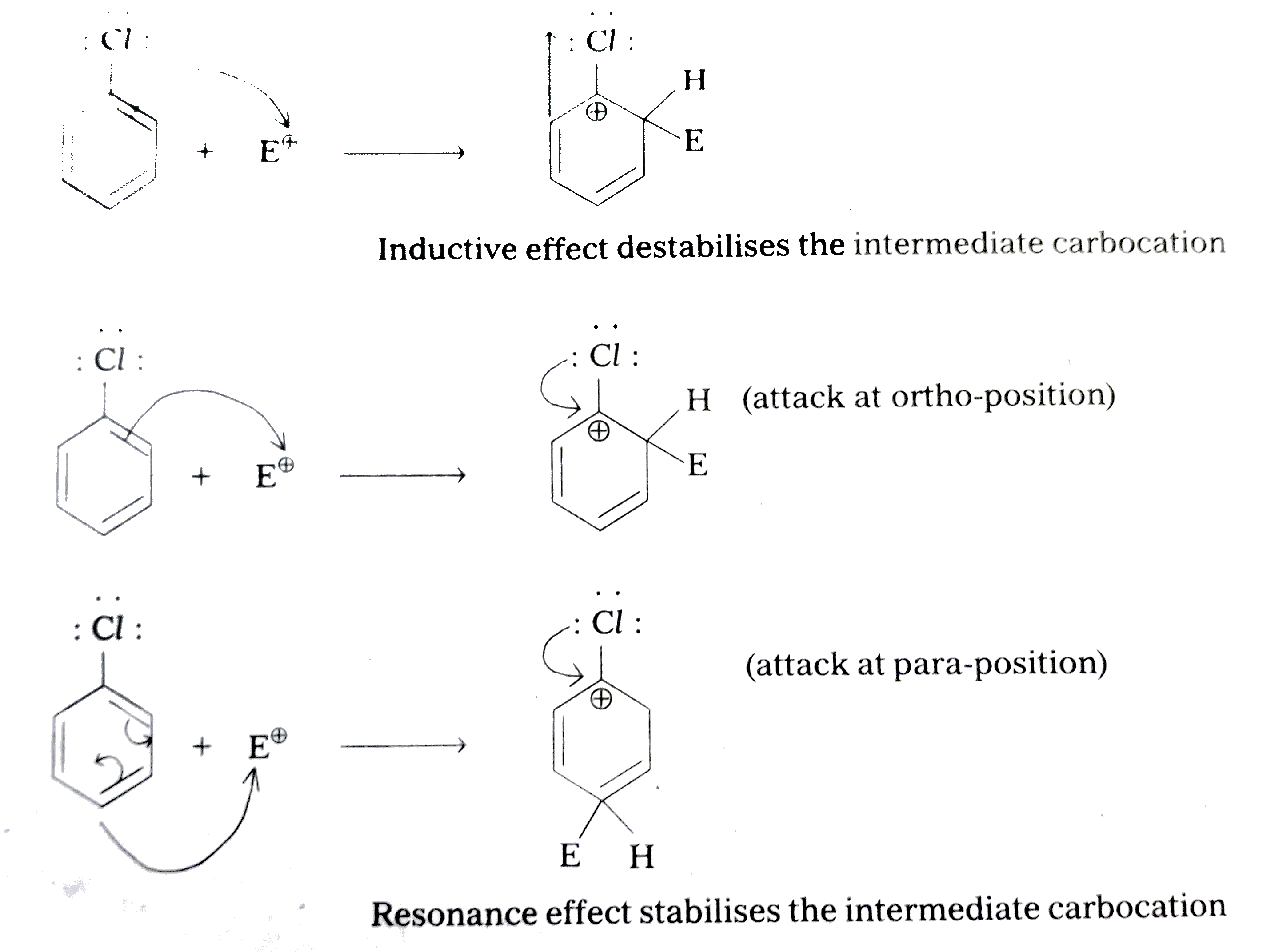
Through resonance , halogen tends to stabilise the carbocation and the effect is more pronounced at ortho and para-positions . The inductive effect is stronger than resonance and causes net electron withdrawl and thus causes net deactivation . The resonance effect trends to oppose the inductive effect for the attack at ortho-and para-positions and hence makes the deactivation less for ortho-and para-attack . Reactivity is thus controlled by the stronger inductive effect and orientation is controlled by resonance effect .
Similar Questions
(a) Why are alkyl halides insoluble in water ? (b) Why is Butan-1- ol optically inactive but Butan-2-ol is optically actove ? (c ) Although chlorine is an electron-withdrawing group, yet it is ortho-para-directing in electrophilic aromatic substitution reactions. Why?
View Solutionक्लोरीन यद्यपि इलैक्ट्रॉन अपचयक समूह है फिर भी यह एरोमैटिक इलैक्ट्रॉनरागी प्रतिस्थापन अभिक्रियाओं मे आर्थो तथा पैरा निर्देशक है, क्यों?
View SolutionThough Chlorine is an electron withdrawing group but it activate the ortho & para position in the benzene ring for electrophilic attack. Explain.
View SolutionExplain why alkoxy group (-OR) is ortho, para directing and activates the aromatic ring towards electrophilic substitution.
View SolutionExplain how the alkoxy group (-OR) is ortho and para-directing and activates the aromatic ring towards electrophilic substitution reactions.
View SolutionAlthough chlorine is an electron withdrawing group, yet is op directing in electrophilic aromatic substitution reaction. Explain.
View SolutionAlthough amino group ls-o- and p- directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nltro aniline. Why?
View SolutionExplain why amino group is ortho para directing in electrophilic substitution reactions .
View Solution
