Write the mechanism of dehydrohalogenation of 2-bromobutane .Solution in Telugu
Video Solution
Text Solution
Dehydrohalogenation of 2-Bromobutane : 2- Bromobutane reacts with alc. KOH to form 2-Butene as major product .
CH3−Br∣CH−CH2−CH3+alc.KOH→CH3−CH=CH−CH32−Butene (major)+KBr+H2O
Mechanism :
→ 2-Bromobutane heated with alc. KOH elemination of hydrogen atom from β-carbon and bromine atom α-carbon takes place . This is called β -Elimination .
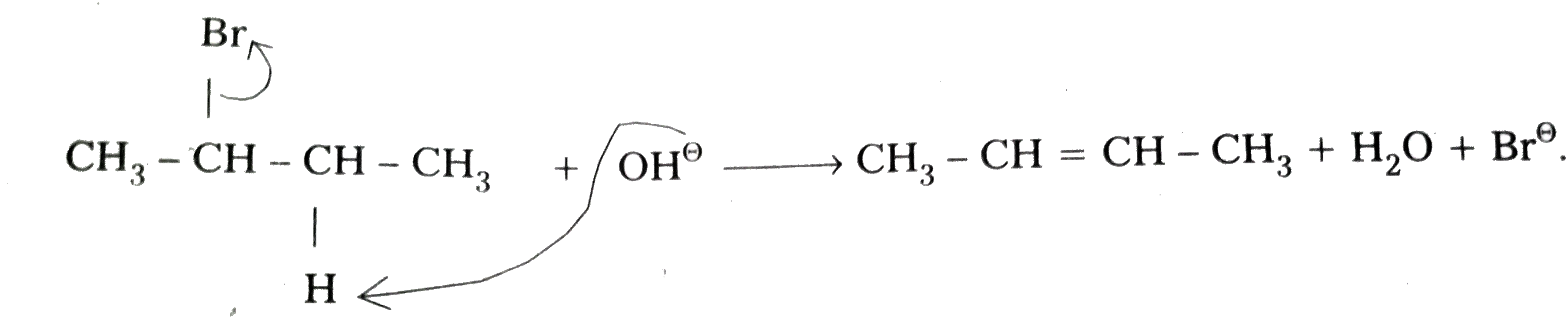
→ 2-Butene is major formed according to saytzev's rule . "In dehydrohalogenation reactions the preferred product is that alkene which has greater no. of alkyl groups attached to double bonded carbon atoms . "
|
Similar Questions
Write the structures of the following organic halogen compounds. 2-Bromobutane
View SolutionExplain the mechanism of reaction of dehydrohalogenization of an alkyl halide.
View SolutionWrite 2-bromobutane in various form
View SolutionWrite 2-bromobutane in various form
View SolutionWrite the bond line structures of 2-Bromobutane
View SolutionWrite the complete, condensed and bondline structural formulae of 2-Bromobutane.
View SolutionReaction Mechanism : elimination reactions,E2 reaction and characteristics(Dehydrohalogenation)
View SolutionConvert :
a. 1−Bromobutane to 2−bromobutane
View Solution

