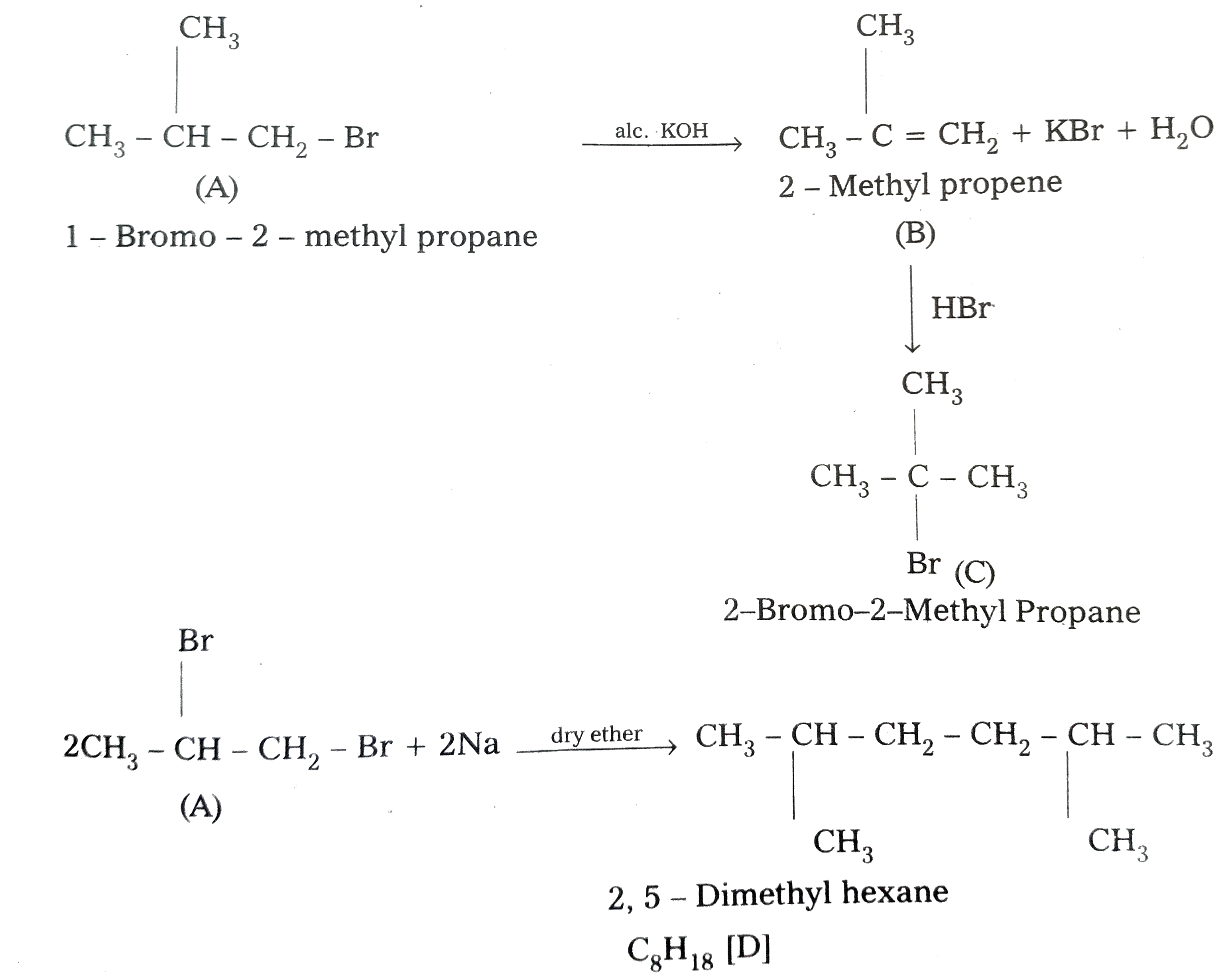
A primary alkyl halide C4H9Br (A) reacted with alcoholic KOH to give compound B . B on reaction with HBr yields C which is an isomer of A . When A is reacted with sodium metal forms D,C6H8 which is different from the compound formed when n-butylbromide is reacted with sodium . Give the structural formulae of A-D and write equations for all the reactions . Solution in Telugu
Given 1∘ -alkyl halide molecular formula C4H9Br
Two isomers possible with molecular formula C4H9Br(i-alkyl halides )
CH3−CH2−CIH2−CH2−Br and CH3−CH3∣CIIH−CH2−Br
→ Given that compound A when reacted with Na does not forms the same product produced by n-Butyl bromide .
∴ isomer I cannot be 'A' .
2CH3−CH2−CH2−n−Butyl bromideCH2−Br+2NaEther−−→C8H18D−Octane
(Given n- actane does not formed in the reaction)
So isomer II must be A
Similar Questions
प्राथमिक से ऐल्किल हैलाइड C4H9Br(A), (एल्कोहोलिक KOH से अभिक्रिया द्वारा यौगिक (B) देता है। यौगिक B,HBr एक साथ अभिक्रिया से यौगिक C देता है जो की यौगिक A का समायवी है। जब यौगिक 'A' की अभिक्रिया से सोडियम धातु से होती है तो यौगिक 'D'C8H18 बनाता है, जो की ब्युटीलब्रोमाइड की सोडियम से अभिक्रिया द्वारा बने उत्पाद से भिन्न है। यौगिक A का संरचना सूत्र दीजिए तथा सभी अभिक्रियाओं की समीकरण दीजिए
View SolutionPrimary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
View Solutionप्राथिमक से ऐल्किल हैलाइड C4H9Br(A), एल्कोहॉलीक KOH से अभिक्रिया द्वारा यौगिक (B) देता है। यौगिक 'B'HBr के साथ अभिक्रिया से यौगिक 'C' देता है जो की यौगिक 'A' का समायवी है। जब यौगिक 'A' की अभिक्रिया सोडियम धातु से होती है यौगिक 'D'C8H18 बनाता है जो की ब्युटीलब्रोमाइड की सोडियम से अभिक्रिया द्वारा बने उत्पाद से भिन्न है। यौगिक 'A' का संरचना सूत्र दीजिए तथा सभी अभीक्रियाओं की समीकरण दीजिए ।
View SolutionPrimary alkyl halide C4H9Br (A) reacted with alcoholic KOH to give compound (B). Compound (B) is reacted with HBr to give (C) which is an isomer of (A). When (A) is reacted with sodium metal it gives compound (D),C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (A) and write the equations for all the reactions.
View Solution(i) A primary alkyl halide (A), C_(4)H_(9)Br reacted with hot alcoholic KOH to give compound (B). Compound (B) reacted with HBr to give (C), which is an isomer of (A). When (A) was reacted with sodium metal, it gave a compound (D), C_(8)H_(18) which was different than the compound when n-butyl bromide was reacted with sodium. Give the structural formula of (A) and write equations of all the reactions. (ii) Iodoform gives a precipitate with AgNO_(3) on heating while CHCl_(3) does not. ? Why ?
View Solutionप्राथमिक ऐल्किल हैलाइड C4H9Br(A), ऐल्कोहॉलिक KOH में अभिक्रिया द्वारा यौगिक (B) देता है | यौगिक (B) HBr के साथ अभिक्रिया से यौगिक (C) देता है जो कि यौगिक (A) का समावयवी है | जब यौगिक (A) की अभिक्रिया सोडियम धातु से होती है तो यौगिक (D) C8H18 बनता है, जो कि n-ब्यूटिल ब्रोमाइड की सोडियम से अभिक्रिया द्वारा बने उत्पाद से भिन्न है | यौगिक (A) का संरचना सूत्र दीजिए तथा सभी अभिक्रियाओं के समीकरण दीजिए |
View SolutionPrimary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
View SolutionPrimary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal, it gives a compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
View Solution

