Explain the acidic nature of phenol.Solution in Telugu
Video Solution
Text Solution
The reaction of phenol with sodium metal and with aq. NaOH indicates the acidic nature of phenol.
i) Phenol reacts with sodium metal to form sodium phenoxide.
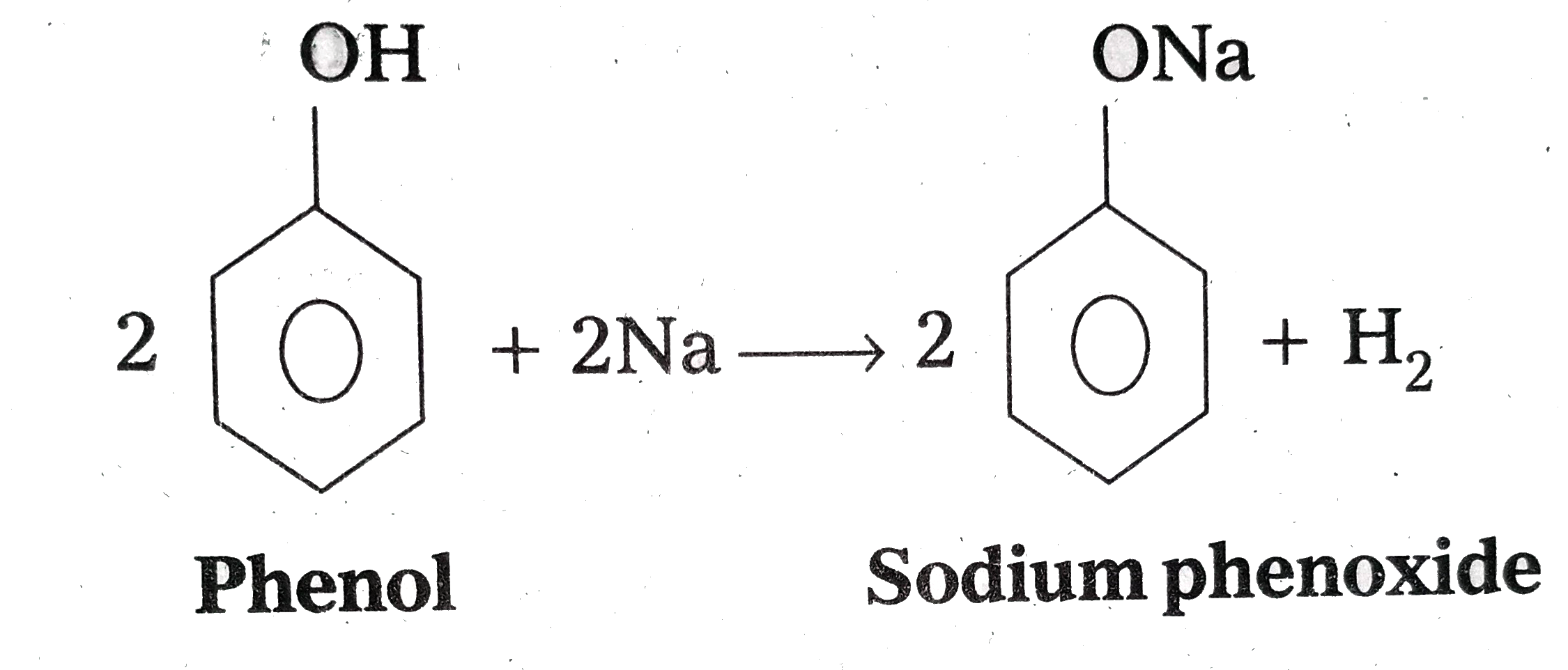
ii) Phenol react with aq. NaOH and form sodium phenoxide.
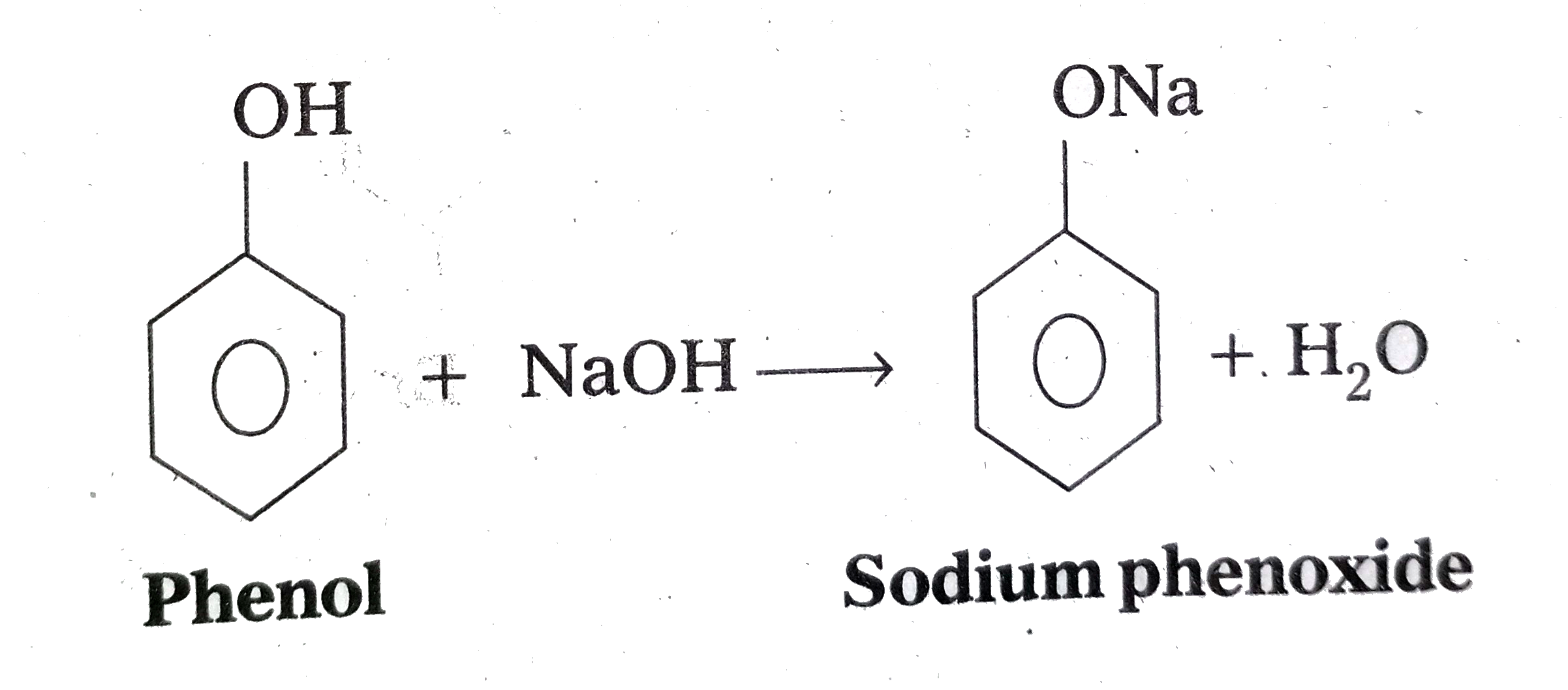
→ phenol hydroxyl group is attached to the Sp2 hydridised carbon of benzene ring which acts as electron with drawing group. The formed phenoxide ion from phenol is more stabilised due to delocalisation of negative charge.
|
Similar Questions
Why phenol is acidic in nature?
View SolutionExplain the acidic nature of phenol.
View SolutionExplain the acidic nature of phenol and explain the reason for the stability of phenoxide ion.
View SolutionBriefly explain the acidic nature of phenols.
View SolutionBriefly explain the acidic nature of phenols.
View Solution(a) Why acidic nature of alcohol and phenol increases with electron with electron withdrawing substituent ? (b) Explain the order of acidic nature of
View SolutionExplain why: Phenols are acidic in nature.
View SolutionWhy phenol is acidic in nature ?
View Solution

