Explain why Aldehydes and ketones undergoes nucleophilic addition while alkenes undergoes electrophilic addition though both are unsaturated compounds.Solution in Telugu
The carbon-oxygen double bond in carbonyl compounds is polarised due to higher electronegativity of oxygen relative to carbon. Hence the carbonyl carbon is an electrophilic and carbonxyl oxygen is a nucleophilic addition reaction.
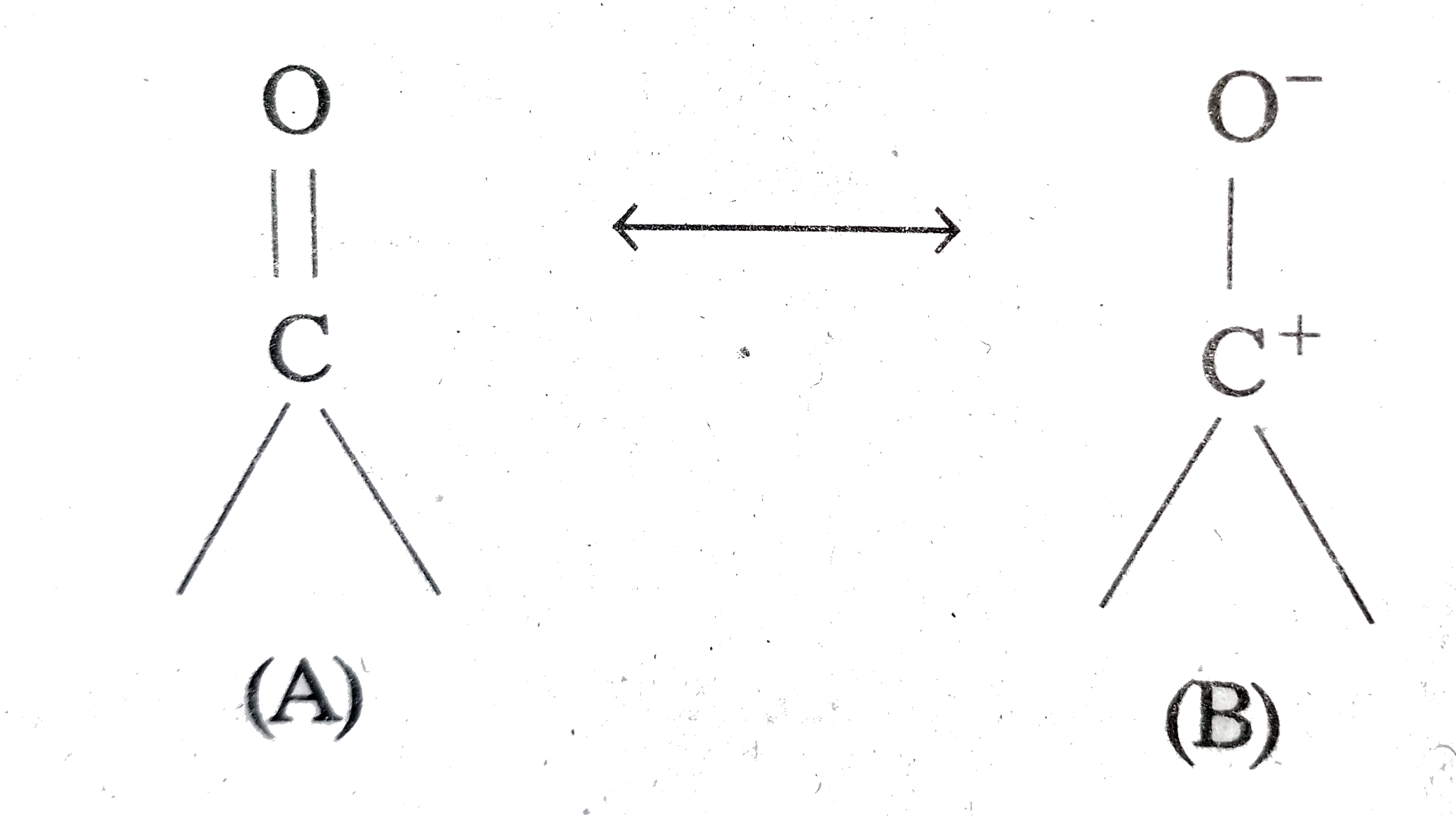
→ Alkenes contain 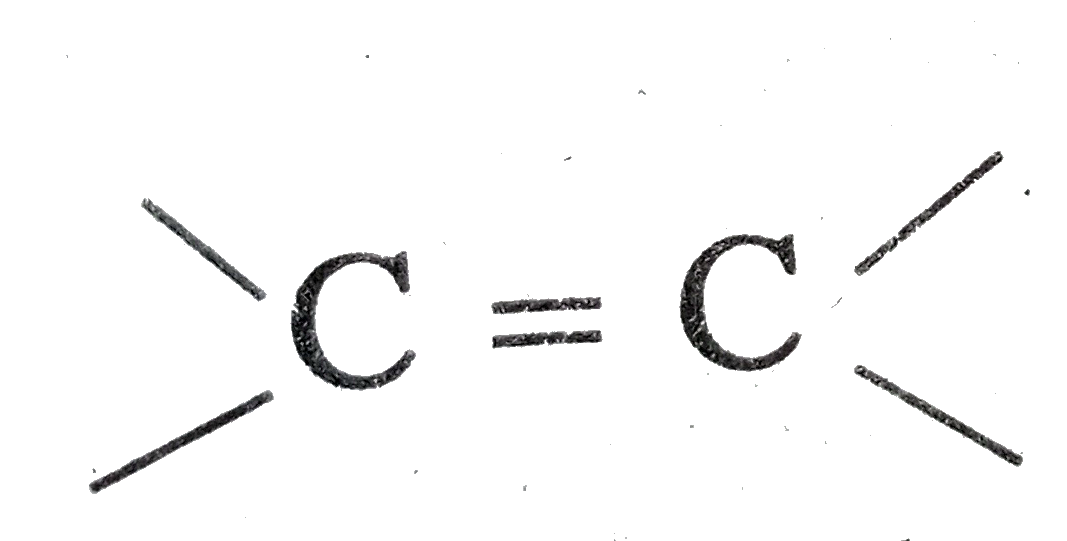 which is a source of electron density to electrophilies add no to C =C to give addition products. Hence alkenes undergo electrophilic addition reaction.
which is a source of electron density to electrophilies add no to C =C to give addition products. Hence alkenes undergo electrophilic addition reaction.
Similar Questions
Why do alkenes prefer to undergo electrophilec addition reaction while arenes prefer electrophilic substitution reactions ? Explain.
View SolutionAlkenes and carbonyl compounds both contain a pi bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
View SolutionWhy do aldehydes and ketones undergo nucleophilic addition reaction?
View SolutionWhy do aldehydes and ketones undergo nucleophillic addition recations ? Give two example each
View SolutionGive reasons for the following observations. Aldehydes and ketones undergo nucleophilic addition reactions.
View SolutionWhy do alkenes undergo electrophilic addition reactions ? Discuss the mechanism of electrophilic addition reactions of alkenes.
View SolutionA : Aldehydes and ketones undergo nucleophilic addition reaction.
R : Aldehydes and ketones contain carbonyl group.
View SolutionWhy do aldehydes and ketones undergo nucleophilic addition reactions. Give one example.
View Solution
