Explain with a suitable example how benzene sulphonylchloride can distinguish primary, secondary and tertiary amines.Solution in Telugu
Video Solution
Text Solution
Benzene sulphonyl chloride is called Hinsberg's reagent. This is used to distinguish the 1∘,2∘,3∘ - amines.
→ with 1∘ - amine : Benzene sulphonyl chloride reacts with 1∘ - amine and produce N - Alkyl benzene sulphonamide which is soluble in alkali.
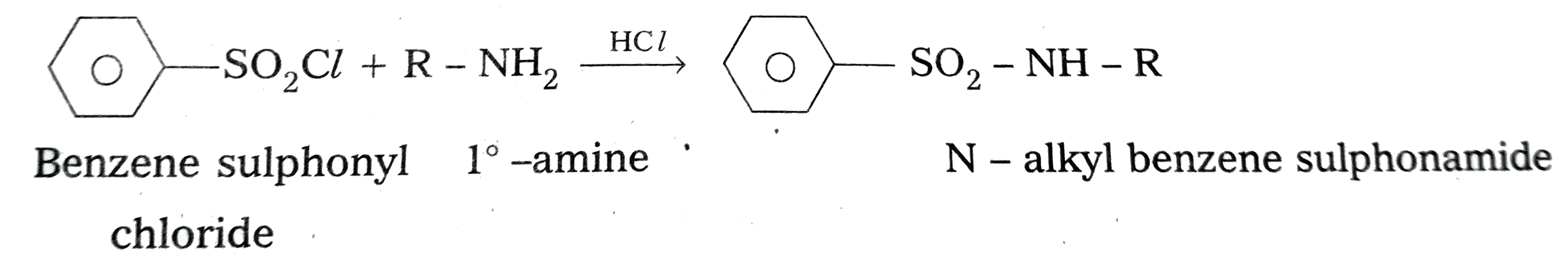
→ with 2∘ - amine : Benzene sulphonyl chloride reacts with 2∘ - amine and produce N, N - Dialkyl benzene sulphonamide which is insoluble in alkali
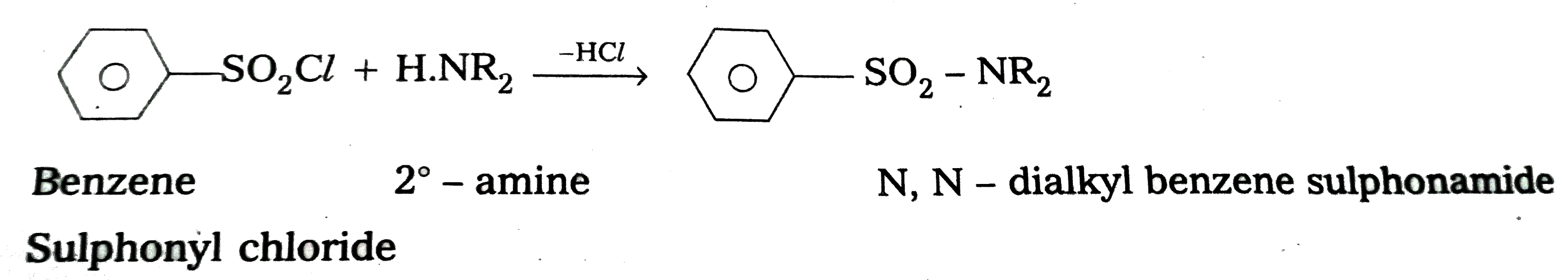
→ with 3∘ - amine : Benzene sulphonyl chloride does not react with benzene sulphonyl chloride.
|
Similar Questions
Primary, secondary and tertiary amines can be distinguished by
View SolutionPrimary, secondary and tertiary amines cannot be distinguished by the action of
View SolutionPrimary aliphatic amines can be distinguished from secondary and tertiary amines by reaching with
View SolutionThe test to distinguish primary, secondary and tertiary amine is
View SolutionPrimary aliphatic amines can be distinguished from secondary and tertiary arnmes by heating with
View SolutionExplain how the three types of alcohols, primary, secondary and tertiary can be distinguished ?
View SolutionHow primary amines are distinguished from secondary and tertiary amines?
View SolutionName the test with which primary, secondary and tertiary amines can be distinguished.
View Solution
