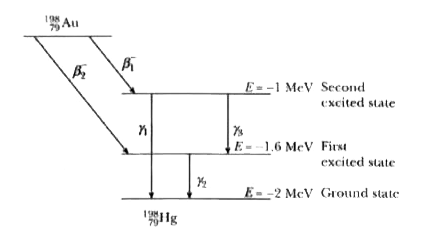
Obtain the maximum kinetic energy of β- particles, and the radiation frequencies of γ decays in the decay scheme shown in Fig. You are given that
m(.198Au)=197.968233u
m(.198Hg)=197.966760u.
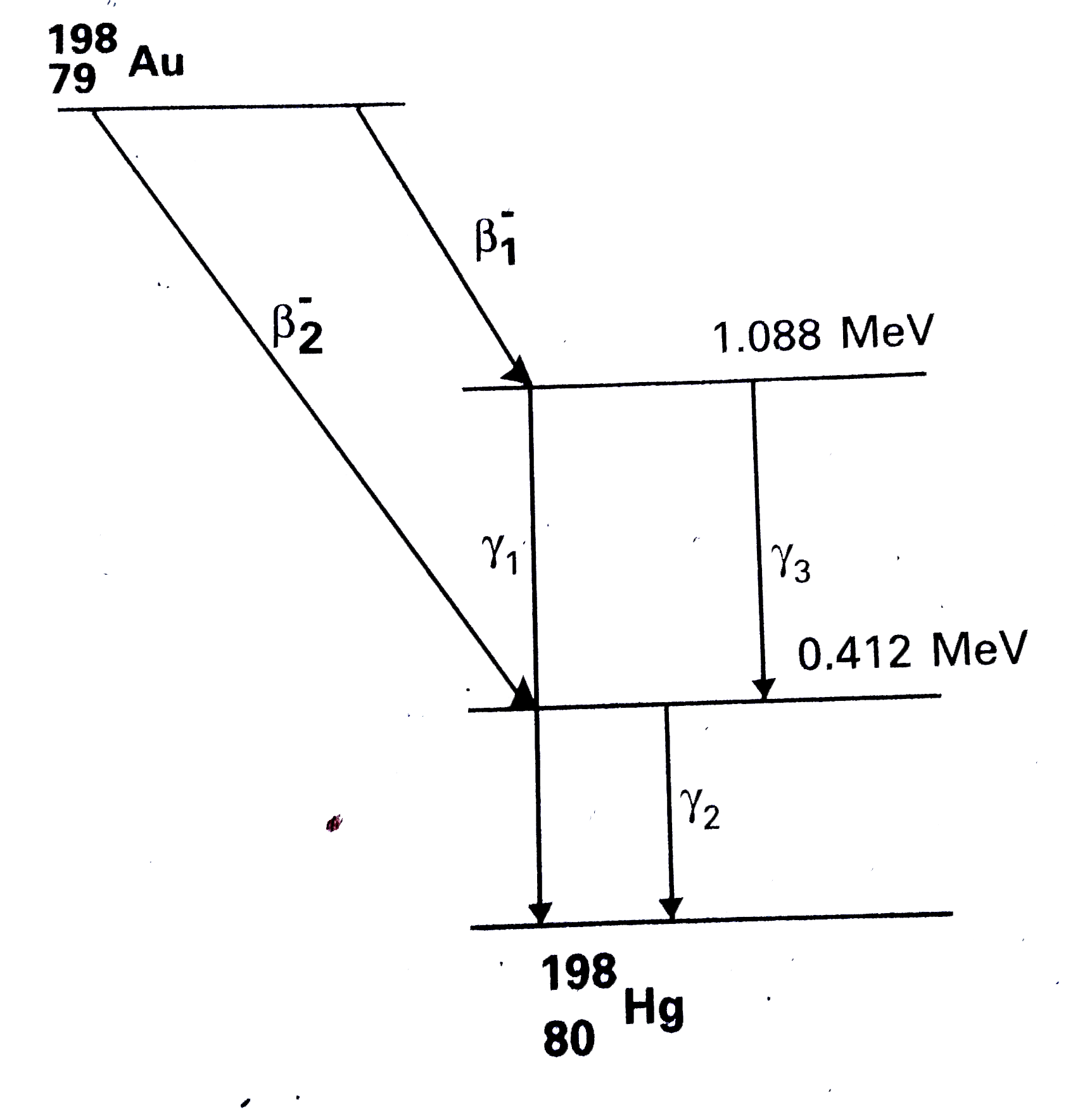
Energy corresponding to r1
E1=1.088−0=1.088MeV
=1.088×1.6×10−13 Joule
Frequency v1=E1h
=1.088×1.6×10−136.6×10−34
=2.63×1020Hz
similary v2=E2h
=0.412×1.6×10−126.6×1010
=9.98×1013Hz
and v3=E3h
=(1.088−0.412)×1.6×10−136.6×1020Hz
Maximum K.E. of β1 particle
Kmax(β1)=[m(.79Au198)− mass of second excited state of .80Hg198]×931MeV
=[m(.79Au198)−m(.82Hg198)−1.088931]×931MeV
=931[197.968233−197.966760]−1.088MeV
=1.371−1.088=0.283MeV
similarly kmax(β2)=0.957MeV
Topper's Solved these Questions
- NUCLEIBOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - NUCLEIEXERCISE - DAM SURE (VSAQ)
1 Video
- NUCLEIBOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - NUCLEIEXERCISE - TEXTUAL EXERCISES
32 Videos
- MOVING CHARGES AND MAGNETISHMBOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - MOVING CHARGES AND MAGNETISHMEXERCISE - ADDITIONAL EXERCISES
26 Videos
- RAY OPTICAL AND INSTRUMENTSBOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - RAY OPTICAL AND INSTRUMENTSEXERCISE - TEXTUAL EXERCISES
60 Videos
Similar Questions
The half life of .58Co is 72 days. Calculate its average life.
View SolutionTwo stable isotopes of lithium .63Li and .73Li have respective abundances of 7.5% and 92.5%. These isotopes have masses 6.01512 u and7.01600 u, respectively. Find the atomic mass of lithium.
View SolutionTwo concentric circular coils X and Y of radii 16 cm and 10 cm, respectively, lie in the same vertical plane containing the north to south direction. Coil X has 20 turns and carries a current of 16 A, coil Y has 25 turns and carries a current of 18 A. The sense of the current in X is anticlockwise, and clockwise in Y, for an observer looking at the coils facing west. Give the magnitude and direction of the net magnetic field due to the coils at their centre.
View SolutionA small candal, 2.5 cm in size is placed at 27 cm in front of a concave mirror of radius of curvature 36 cm. At wht distance from the mirror should a screen be placed in order to obtain a sharp image ? Describe the nature and size of the image. If the candale is moved closer to the mirror, how would the screen have to be moved ?
View SolutionConsider the beta decay
^198Au→198Hg∗+Bη−1+→v.
where ^198Hg∗ represents a mercury nucleus in an excited state at energy 1.088MeV above the ground state. What can be the maximum kinetic energy of the electron emitted? The atomic mass of ^198Au is 197.968233u and that of ^198Hg is 197.966760u.
View SolutionThe half-life of ^198Au is 2.7days. Calculate (a) the decay constant, (b) the average-life and (C ) the activity of 1.00mg of ^198Au. Take atomic weight of ^198Au to be 198gmol−1.
View SolutionGold ._(79)^(198)Au undergoes beta^(-) decay to an excited state of ._(80)^(198)Hg . If the excited state decays by emission of a gamma -photon with energy 0.412 MeV , the maximum kinetic energy of the electron emitted in the decay is (This maximum occurs when the antineutrino has negligble energy. The recoil enregy of the ._(80)^(198)Hg nucleus can be ignored. The masses of the neutral atoms in their ground states are 197.968255 u for ._(79)^(198)Hg ).
View SolutionObtain the maximum kinetic energy of β-particles, and the radiation frequencies of γ decays in the decay scheme shown in Fig. 14.6. You are given that m(.198Au)=197.968233u,m(.198Hg)=197.966760u
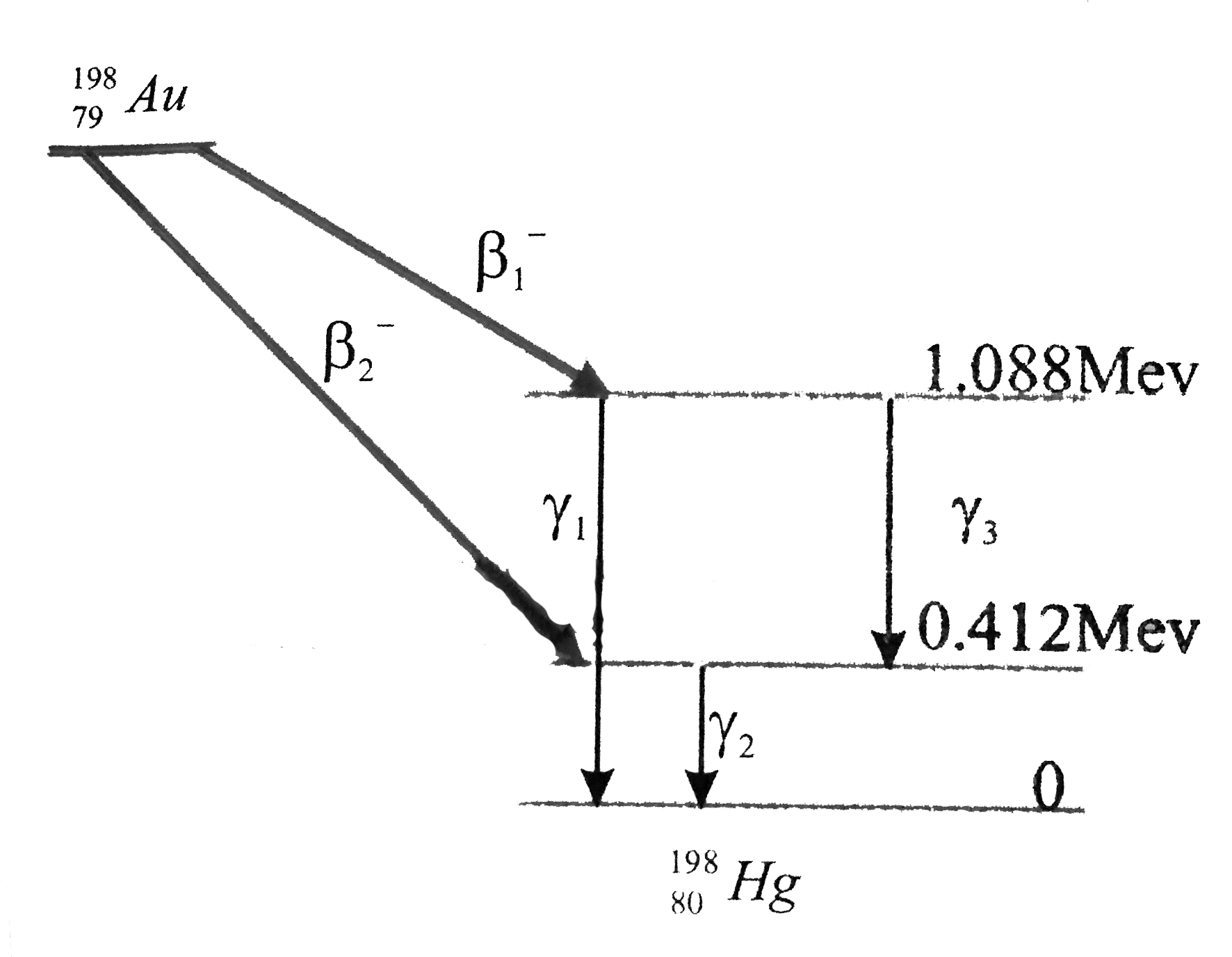
View Solutionनीचे दी गई क्षय योजना में γ क्षयों की विकिरण आवृत्तियां एवं β कणों की अधिकतम गतिज ऊर्जाएं ज्ञात कीजिए। दिया है
m(.198Au)=197.968233u
m(.198Hg)=197.966760u
View SolutionThe nucleus ._(10)^(23)Ne decays by beta - emission. Write down the beta - decay. Write down the beta - decay equation and determine the maximum kinetic energy of the electrons emitted. Given that : m(._(10)^(23)Ne)=22.994466 u m(._(11)^(23)Na)=22.089770 u .
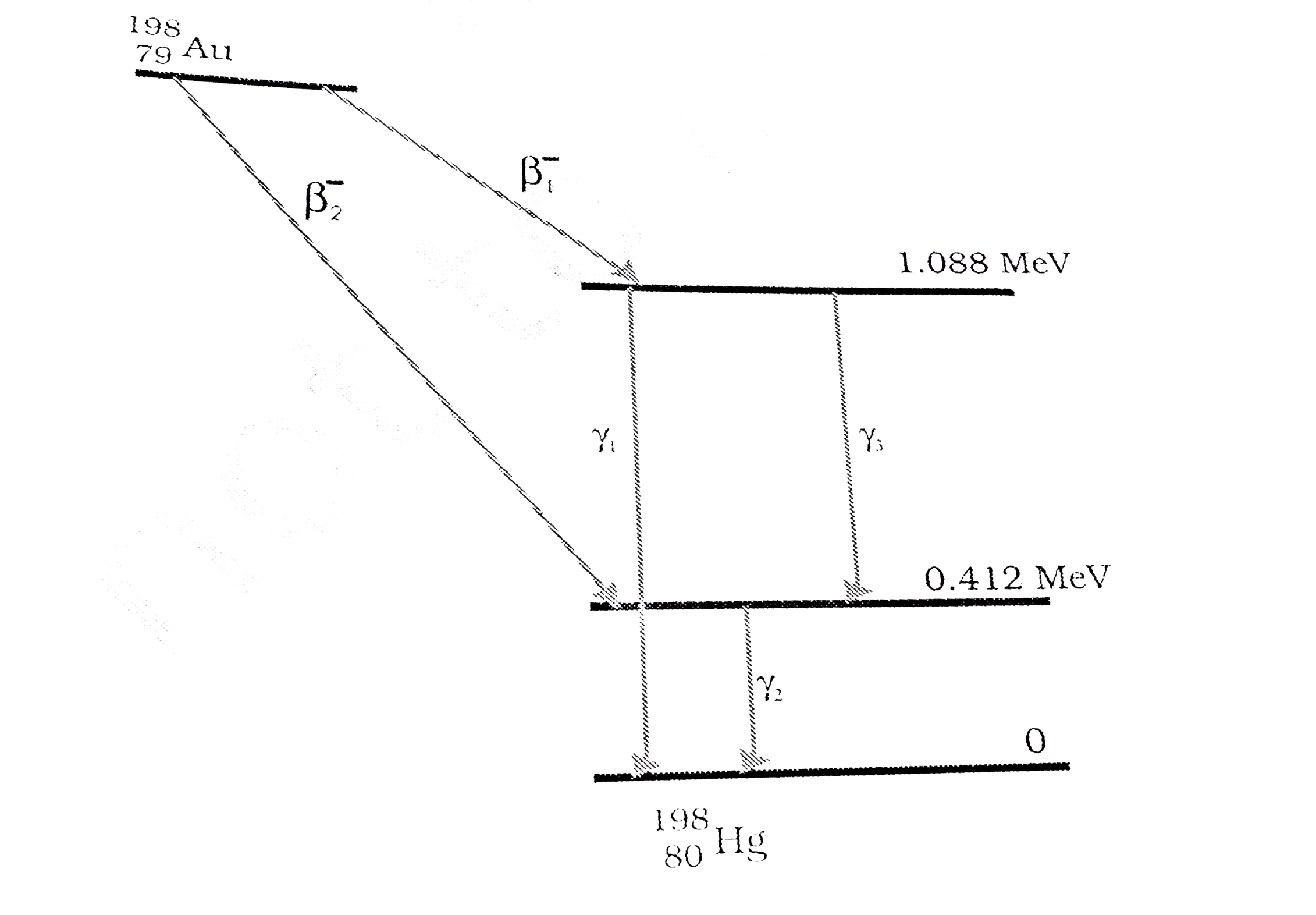
View Solutionनीचे दी गयी क्षय योजना ( decay scheme ) में β - कणो की अधिकतम गतिज उर्जाएँ था γ - कानो की विकिरण आवृत्तियाँ (radiation frequencies ) ज्ञात करे |
दिया गया है :m(19879Au)=197.968233 u
m(19880Hg)=197.966760u .
View SolutionObtain the maximum kinetic energy of β- particle and the radiation frequencies corresponding to γ− decays in the figure given below. (rest mass of au -198.968233 u and r.m. of Hg=198=197.966760 u)
View Solution198Au की अर्ध - आयु 2.7 दिन है | 1.0mg198Au का (a) क्षय स्थिरांक , (b) औसत आयु और (c) सक्रियता निकालें
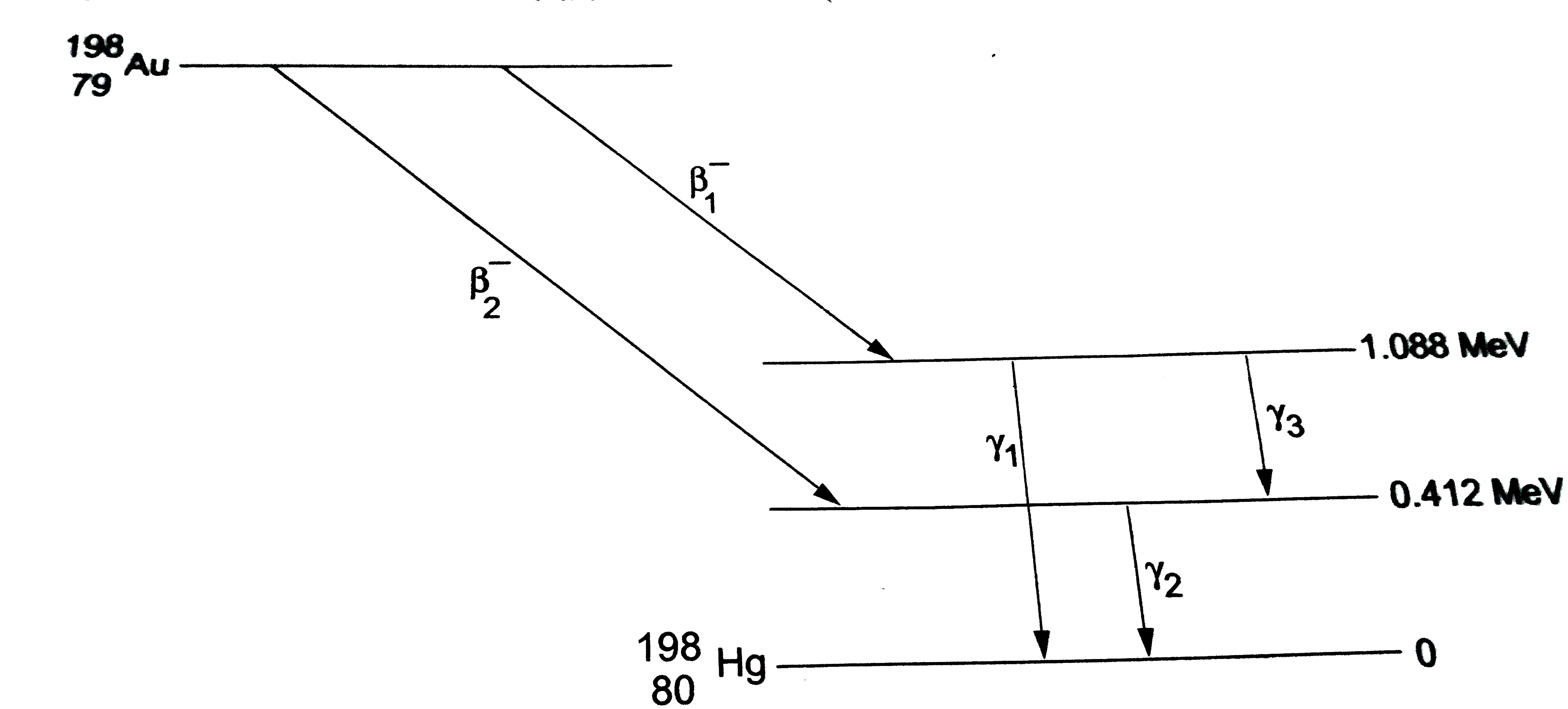
View SolutionObtain the maximum kinetic energy of b-particles, and the radiation frequencies of g decays in the decay scheme shown in Fig. You are given that m(""_(8)^(19)Au) = 197.968233 u m(""_(8)^(19)Hg) =197.966760 u .
View SolutionGold nucleus (""_(79)^(198)Au) can decay into mercuyr nucleus (""_(80)^(198)Hg^(8)) by two decay schemes shown in figure below (i) it can emit a beta -particle (beta_(1)) and come to ground state by either emiting one gamma - ray ( gamma_(1) ) or emitting two gamma - rays ( gamma_(3) and gamma_(4) ) (ii) It can emit one beta - particle (beta_(2)) and come to ground state by emitting gamma_(2) ray. Atomic masses: ""^(198)Au=197.9652 amu and ""^(198)Hg=197.9662 amu. Given that 1 amu= 930MeV//c^(2) . The energy levels of the nucleus are shown in figure below Whatis the maximum kinetic energy of emitted beta_(2) particle?
View SolutionGold nucleus (19879Au) can decay into mercuyr nucleus (19880Hg8) by two decay schemes shown in figure below (i) it can emit a β -particle (β1) and come to ground state by either emiting one γ- ray (γ1) or emitting two γ- rays (γ3 and γ4) (ii) It can emit one β- particle (β2) and come to ground state by emitting γ2 ray. Atomic masses: 198Au=197.9652 amu and 198Hg=197.9662 amu. Given that 1 amu= 930MeV/c2. The energy levels of the nucleus are shown in figure below
What is the maximum kinetic energy of emitted β1 parricle ?
View Solution

