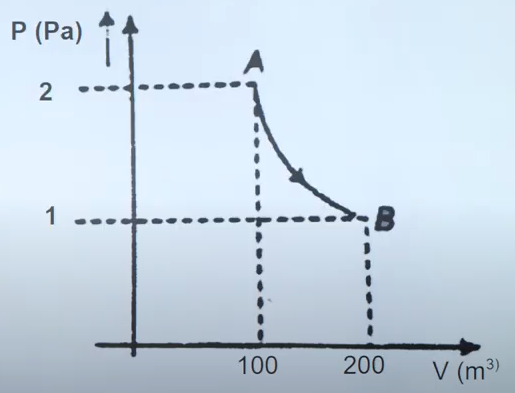
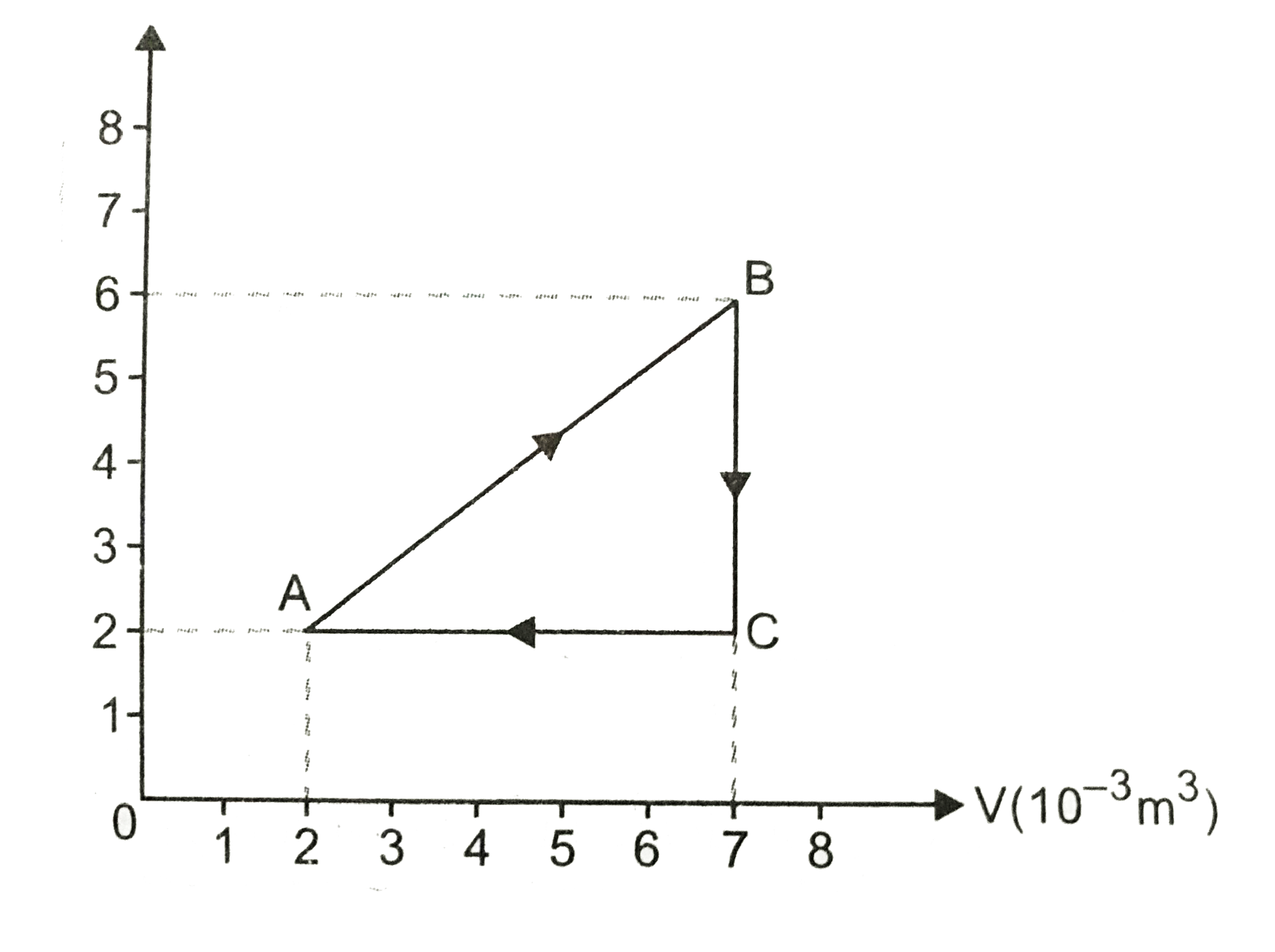
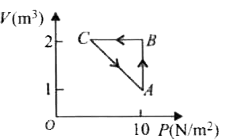
A fixed mass of gas is taken through a process A→B→C→A. Here A→B is isobaric, B→C is adiabatic and C→Ais isothermal.
Find pressure at C.
10564N/m2
10532N/m2
10512N/m2
1056N/m2
The correct Answer is:A
a For adiabatic process BC,
PB=PC ...(i)
For isothermal process CA
PAVA=PCVC ...(ii)
From Eqs. (i) and (ii)
VC=[VγBVA]1γ−1=64m3
PC=PAVAVC=10564N/m2
b Work done, W=WAB+WBC+WCA
=P(VB−VA)+1γ−1[PVB−PCVC+PVA1n(VA/VC)]
Putting the values,
W=4.9×105J
Similar Questions
Knowledge Check
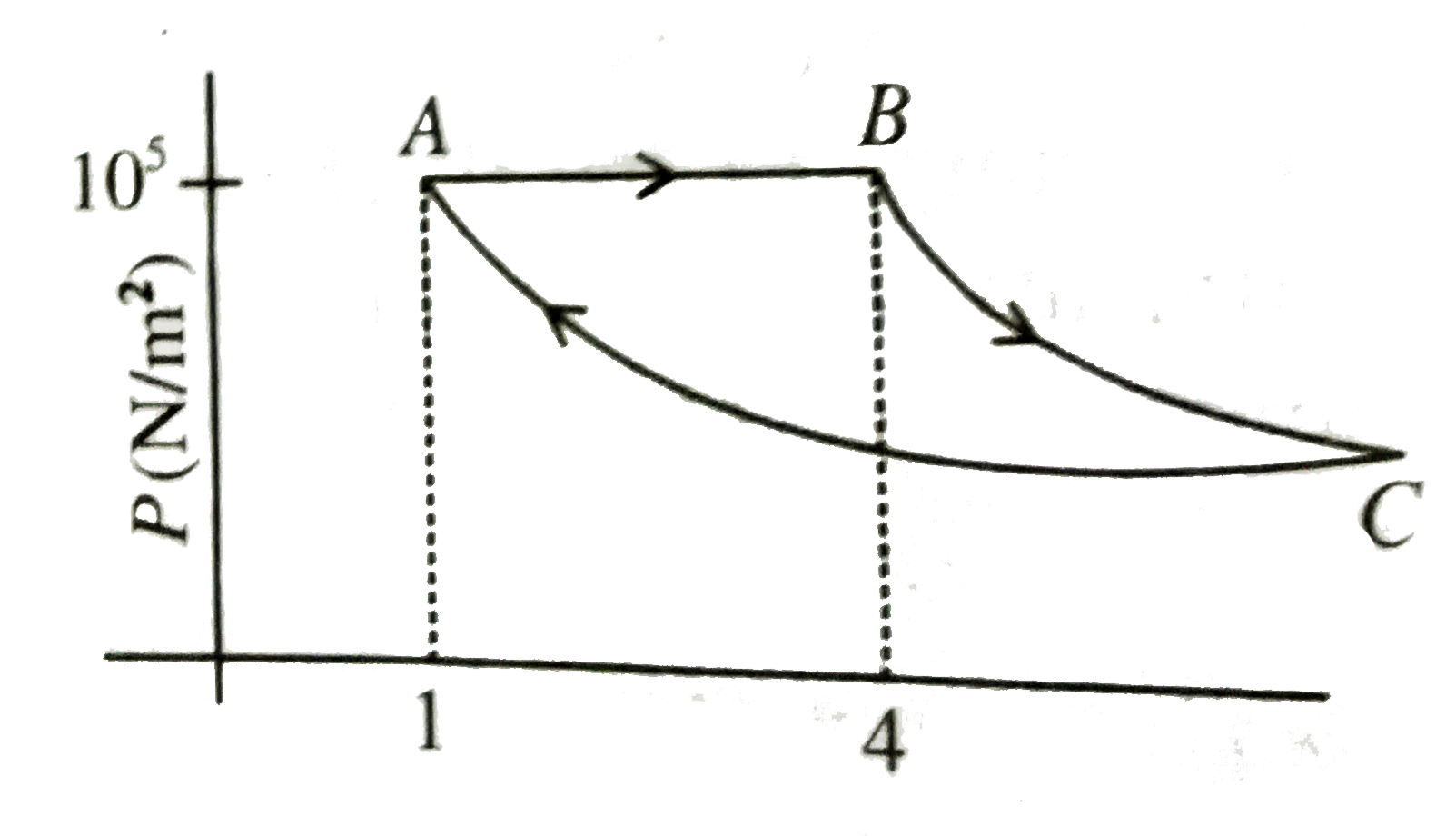
A fixed mass of gas is taken through a process A→B→C→A. Here A→B is isobaric, B→C is adiabatic and C→Ais isothermal.
 Find volume at C. A32m3B100m3C64m3D25m3
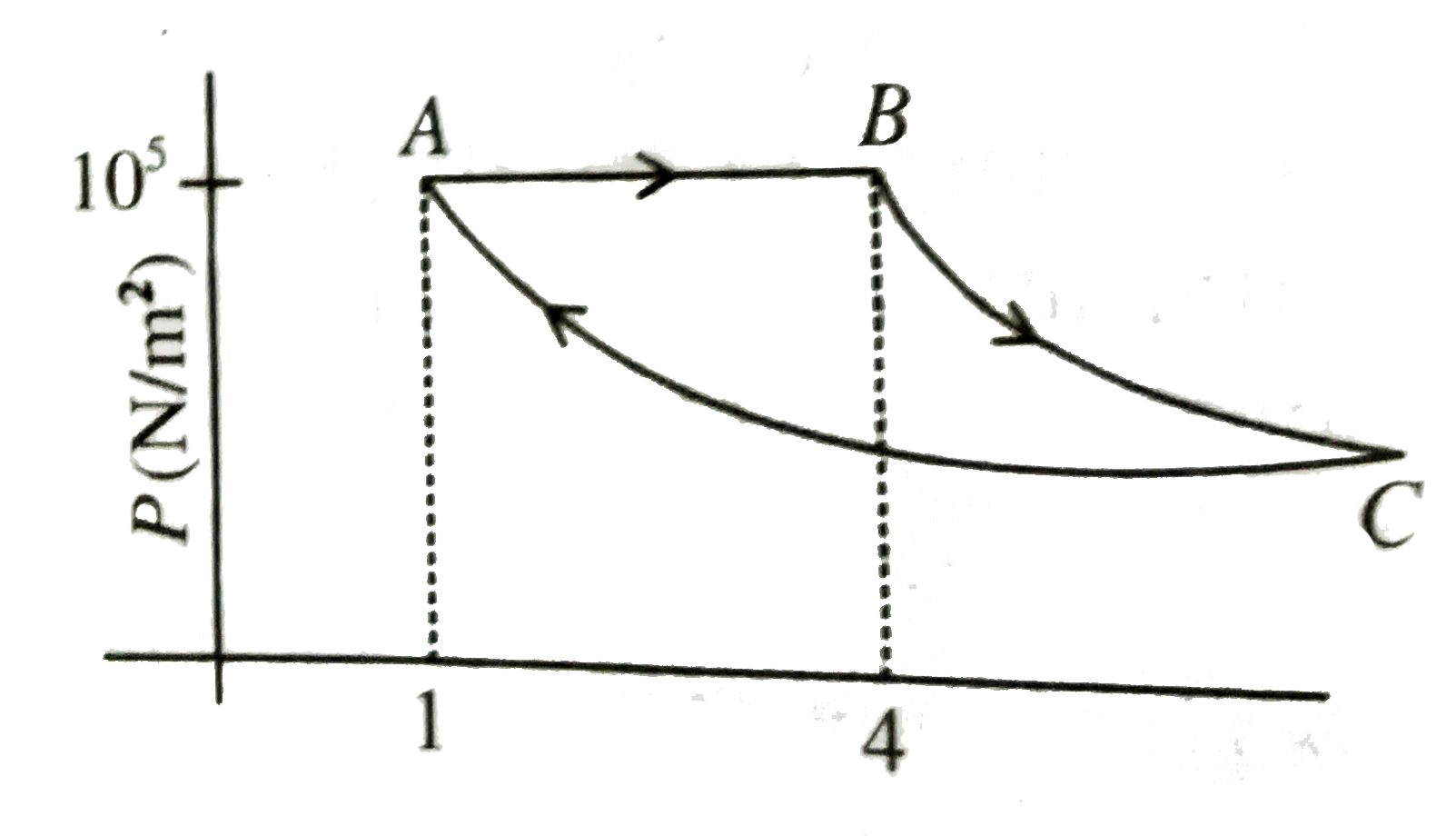
Find volume at C. A32m3B100m3C64m3D25m3A fixed mass of gas is taken through a process A→B→C→A. Here A→B is isobaric, B→C is adiabatic and C→Ais isothermal.
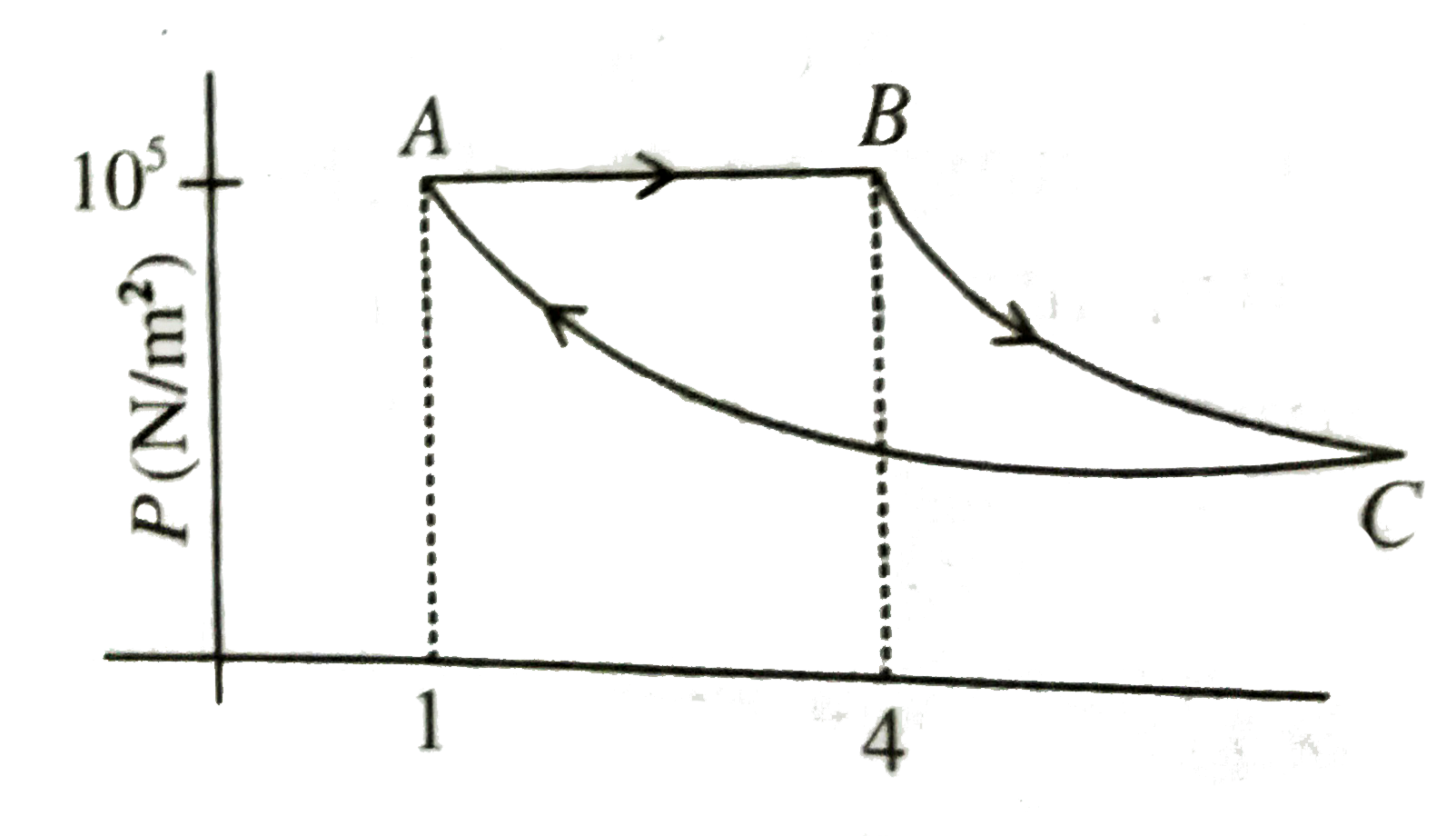
Find work done is the process ( take γ=1.5).A4.9×105JB3.2×105JC1.2×105JD7.2×105JA gas is taken through the cycle A→B→C→A, as shown. What is the net work done by the gas ?
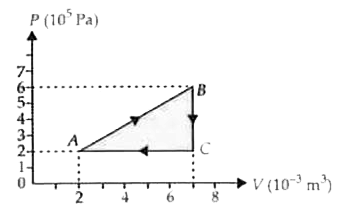 AzeroB−2000JC2000 JD1000 J
AzeroB−2000JC2000 JD1000 J
An ideal gas is taken through the cycle A→B→C→A As shown in figure. If net heat supplied to the gas in the cycle is 5j. Find the work done by the gas in the process C→A in Joule (taken mole value)
View SolutionLet f:A→Bandg:B→C be two functions and gof:A→C is define statement(s) is true?
View SolutionA gas is taken through the cycle A→B→C→A, as shown in figure, what is the net work done by the gas?
View Solution0.2 moles of an ideal gas is taken round the cycle ABC as shown in the figure. The path B→C is an adiabatic process A→B is an isochoric process and C→A is an isobaric process. The temperature at A and B are TA300K and TB=500k and pressure at A is 1 atm and volume at A is 4.91. The volume at C is
(given : γ=CPCV=53,R=8.205×10−2Latm mol−1K−1,(32)2/5=0.81)
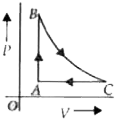
View SolutionAn ideal gas is taken through the cycle A→B→C→A, as shown in figure. If the net heat supplied to the gas in the cycle is 5J, the work done by the gas in the process C→A is
View Solution