Four moles of an ideal gas is initially in state A having pressure 2×105N/m2 and temperature 200K . Keeping the pressure constant the gas is taken to state B at temperature of 400K. The gas is then taken to a state C in such a way that its temperature increases and volume decreases. Also from B to C , the magnitude of dT/dV increases. The volume of gas at state C is eqaul to its volume at statem A. Now gas is taken to initial state A keeping volume constant. A total 1000J of heat is withdrawn from the sample of the cyclic process . Take R=8.3J/K/mol.
Which graph between temperature T and volume V for the cyclic process is correct.
(##BMS_V06_C02_E01_249_O01.png" width="30%">
(##BMS_V06_C02_E01_249_O02.png" width="30%">
(##BMS_V06_C02_E01_249_O03.png" width="30%">
(##BMS_V06_C02_E01_249_O04.png" width="30%">
The correct Answer is:C
An AB is isobaric, in B→C magnitude of dTdv increases and OA is isochoric process.
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICSCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Interger
11 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICSCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Assertion-Reasoning
6 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICSCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICSCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Multiple Correct Answer Type
9 Videos
Similar Questions
The following graphs are plotted for an ideal gas taken from state A to state B and then to state C, maintaining volume constant.
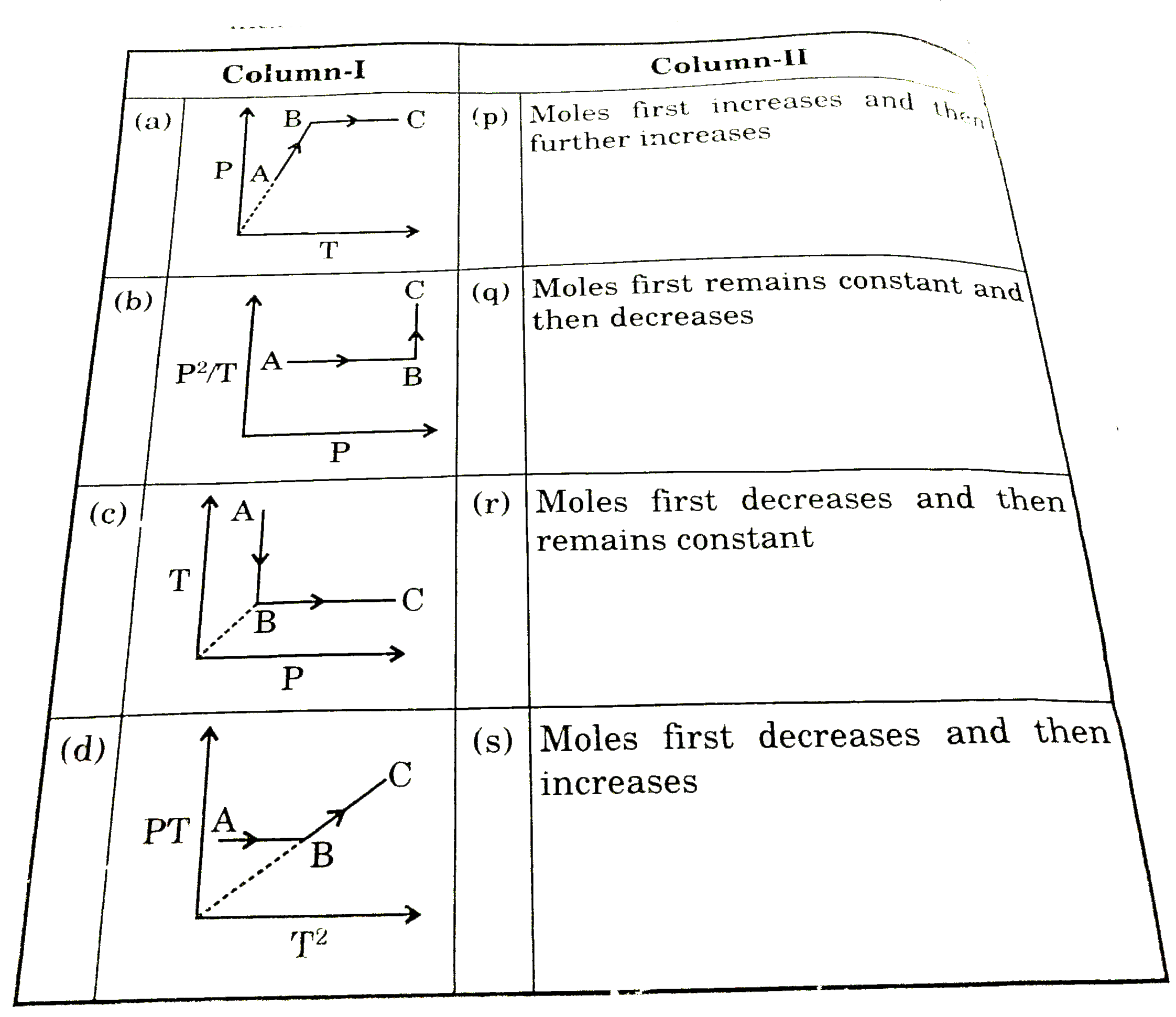
View SolutionOne mole of an ideal monoatomic gas is taken at a temperature of 300K. Its volume is doubled keeping its pressure constant. Find the change in internal energy.
View Solution
Knowledge Check
Four moles of an ideal gas is initially in state A having pressure 2×105N/m2 and temperature 200K . Keeping the pressure constant the gas is taken to state B at temperature of 400K. The gas is then taken to a state C in such a way that its temperature increases and volume decreases. Also from B to C , the magnitude of dT/dV increases. The volume of gas at state C is eqaul to its volume at statem A. Now gas is taken to initial state A keeping volume constant. A total 1000J of heat is withdrawn from the sample of the cyclic process . Take R=8.3J/K/mol. The volume of gas at state C is
A0.0332m3B0.22m3C0.332m3D3.32m3Four moles of an ideal gas is initially in state A having pressure 2×105N/m2 and temperature 200K . Keeping the pressure constant the gas is taken to state B at temperature of 400K. The gas is then taken to a state C in such a way that its temperature increases and volume decreases. Also from B to C , the magnitude of dT/dV increases. The volume of gas at state C is eqaul to its volume at statem A. Now gas is taken to initial state A keeping volume constant. A total 1000J of heat is withdrawn from the sample of the cyclic process . Take R=8.3J/K/mol.
The work done in path B to C isA1000JB−1000JC−7640JD5640JFour moles of an ideal gas is initially in a state A having pressure 2×105N/m2 and temperature 200 K . Keeping pressure constant the gas is taken to state B at temperature of 400K. The gas is then taken to a state C in such a way that its temperature increases and volume decreases. Also from B to C, the magnitude of dTdV increases. The volume of gas at state C is eaual to its volume at state A. Now gas is taken is initial state A keeping volume constant. A total of 1000 J heat is rejected from the sample in the cyclic process. Take R=8.3J/K/mol.
The work done by the gas along path B to C isA1000JB−1000JC−7640JD5640J
The temperature of a certain mass of gas was increased from 40∘C to 41∘C at constant pressure.The volume of the gas
View SolutionFour moles of an ideal gas is initially in a state A having pressure 2×105N/m2 and temperature 200 K . Keeping pressure constant the gas is taken to state B at temperature of 400K. The gas is then taken to a state C in such a way that its temperature increases and volume decreases. Also from B to C, the magnitude of dTdV increases. The volume of gas at state C is eaual to its volume at state A. Now gas is taken is initial state A keeping volume constant. A total of 1000 J heat is rejected from the sample in the cyclic process. Take R=8.3J/K/mol.
Which graph between temperture T and volume V for the cyclic process is correct.
View SolutionTwo moles of an idea gas is initially in state A having pressure 1.01×105N/m2 and temperature 3O0k keeping pressure constant the gas is taken to state B temperature at B is 500K the gas is then taken to state C in such a way that its temperature increases and volume decreases also from B To c the magnitude of dTdV increases the volume of gas at C is equal to volume of gas at A now the gas is taken to initial state A keeping volume constant A total of 1200 J of heat is with drawn from the sample in the cyclic process the T-V graph for the cyclic process and work done in path B to C are respectively(takeR=8.3J/k/mol)
View SolutionThe temperature of a given mass of a gas is increased from 19∘C to 20∘C at constant pressure. The volume V of the gas is
View SolutionOne mole ideal monatomic gas is taken at temperature of 300 K. If volume is doubled keeping its pressure constant. The change in the internal energy is
View Solution

