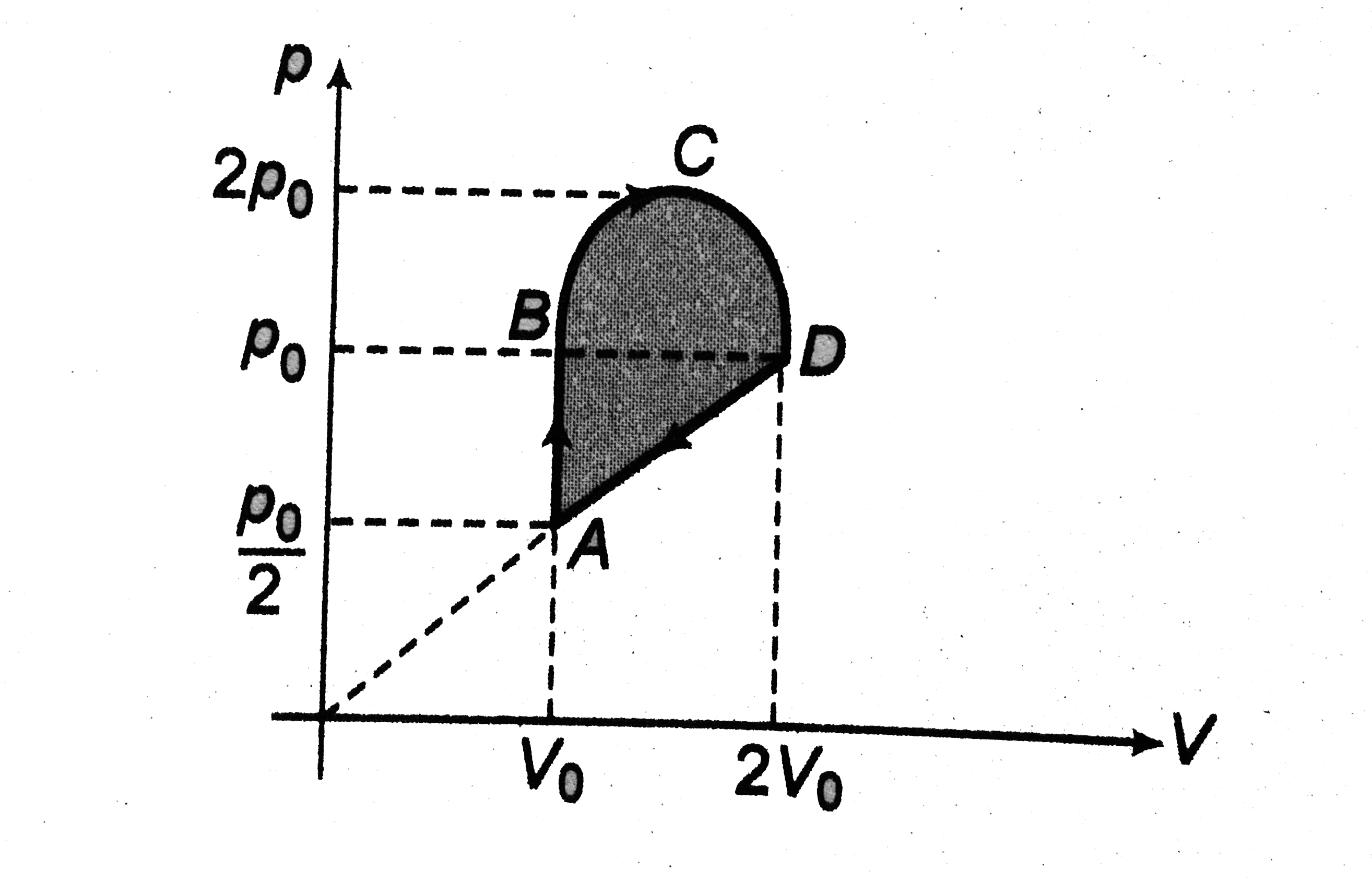
A monatomic ideal gas undergoes the shown cyclic process in which path of the process 2→3 is a semicircle. If 2mol of gas is taken, find

Total heat rejected in one cycle
P0V0(32−π)4
P0V0(32+π)4
P0V0(32+π)8
P0V0(32−π)8
The correct Answer is:D
Process 1→2 ( Isochoric process )
W12=0
Q12=ΔU12=nCV(T2−T1)
=n32R(T2−T1)
=32[P0V0−(P0/2)V0]
=3P0V04 (heat absorbed )
Process 3→1
W3→1= Area under process 3→1
=−(P02+P0).V02=−3P0V04
Work done during 3→1 will be negative as volume is decreasing.
ΔU3→1=n3R2(T1−T2)32(P0V02−P02V0)=−9P0V04
Q3→1=ΔU3→1+W3→1=−3P0V0 ( heat rejected )
process 2→3
The temperature will be maximum at x=2P0,Tx=3P0V02R
Wr→x=Wx→3=14πP0V02+P0V02=P0V02(π4+1)
W2rar3=P0V04(π+4)
ΔU2→x=n32R(Tx−T2)=32(3P0V0−P0V0)=3P0V0
ΔUx→3=n32R(Tx−T2)=32(2P0V0−3P0V0)
=−32P0V0
∴ΔQ2→x=ΔU2→x+ΔW2→x
=3P0V0+P0V02(π4+1)
=P0V0(3+12+π2)=P0V02(7+π4)
ΔQx→3=ΔUx→3+Wx→3
=−32P0V0+P0V02(π4+1)=−P0V02(2−π4)
Total heat rejected in one cycle
=3P0V0+P0V02(2−π4)=4P0V0−πP0V08
=P0V0(4−π8)=P0V0(32−π)8
Similar Questions
Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as shown in figure. BCD is a semicircle. Find the efficiency of the cycle.
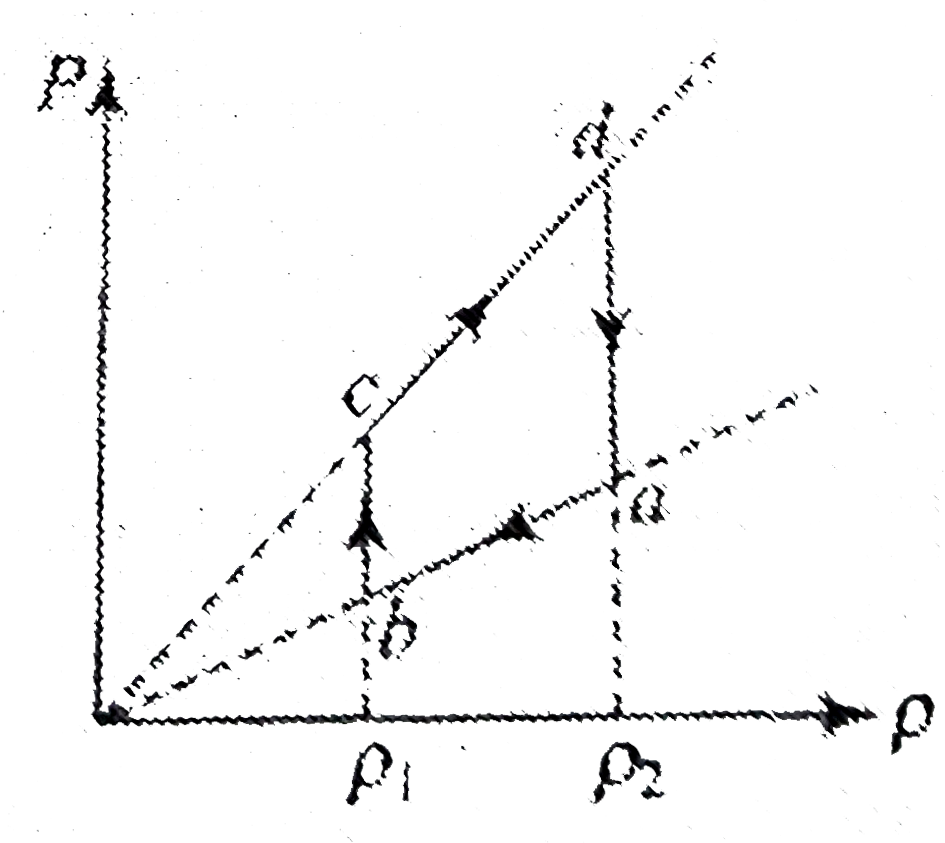
View SolutionThe density (ρ) versus pressure (p) graph of one mole of an ideal monoatomic gas undergoing a cyclic process is shown in figure. Molecular mass of gas is M.
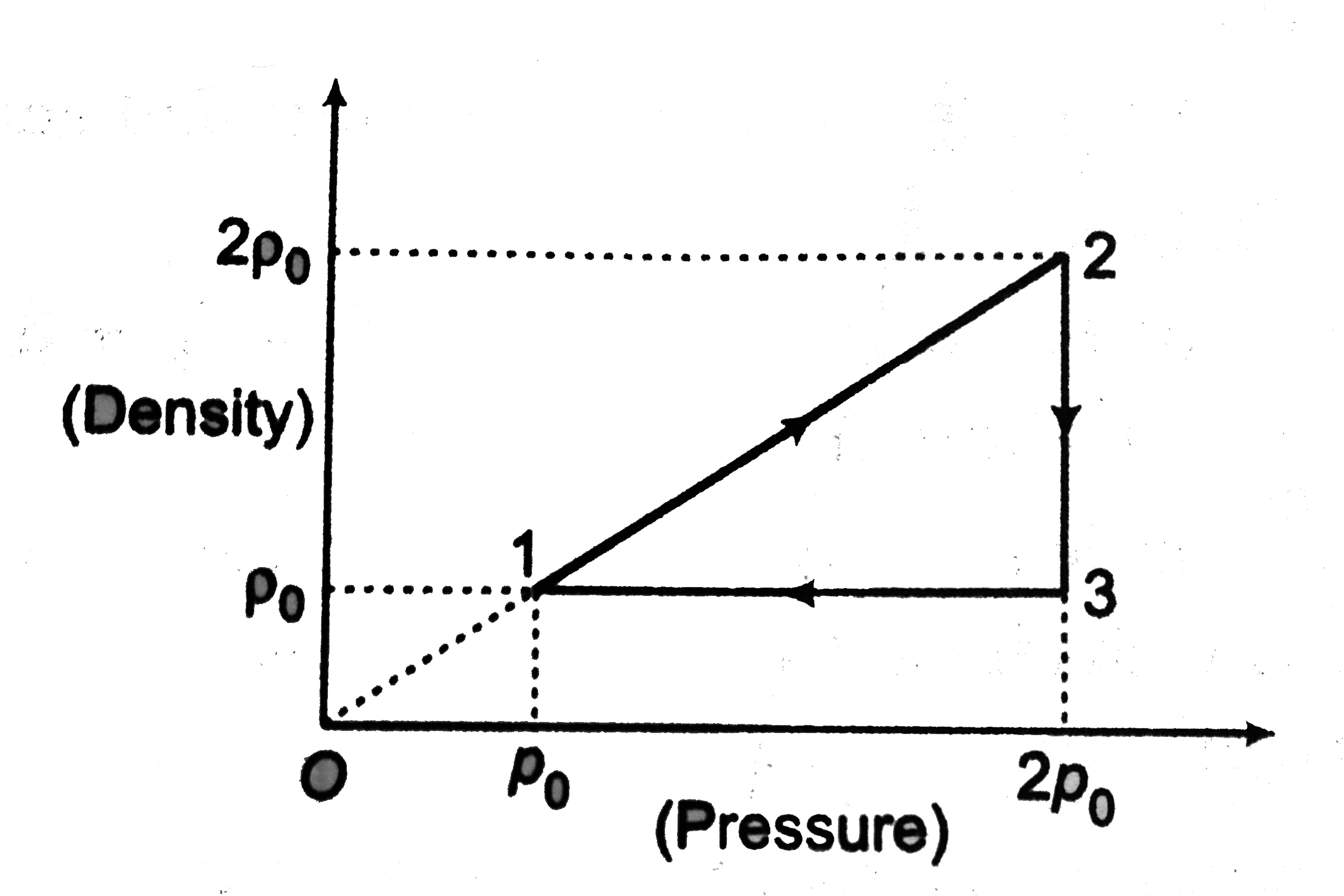
(a) Find work done in each process.
(b) Find heat rejected by gas in one complete cycle.
(c) Find the efficiency of the cycle.
View Solution
Knowledge Check
A monatomic ideal gas undergoes the shown cyclic process in which path of the process 2→3 is a semicircle. If 2mol of gas is taken, find

Heat given to the system in semicirculat processAP0V02(7−π4)BP0V03(7+π4)CP0V02(7+π4)DP0V03(7−π4)An ideal gas undergoes the cyclic process shown in a graph below :
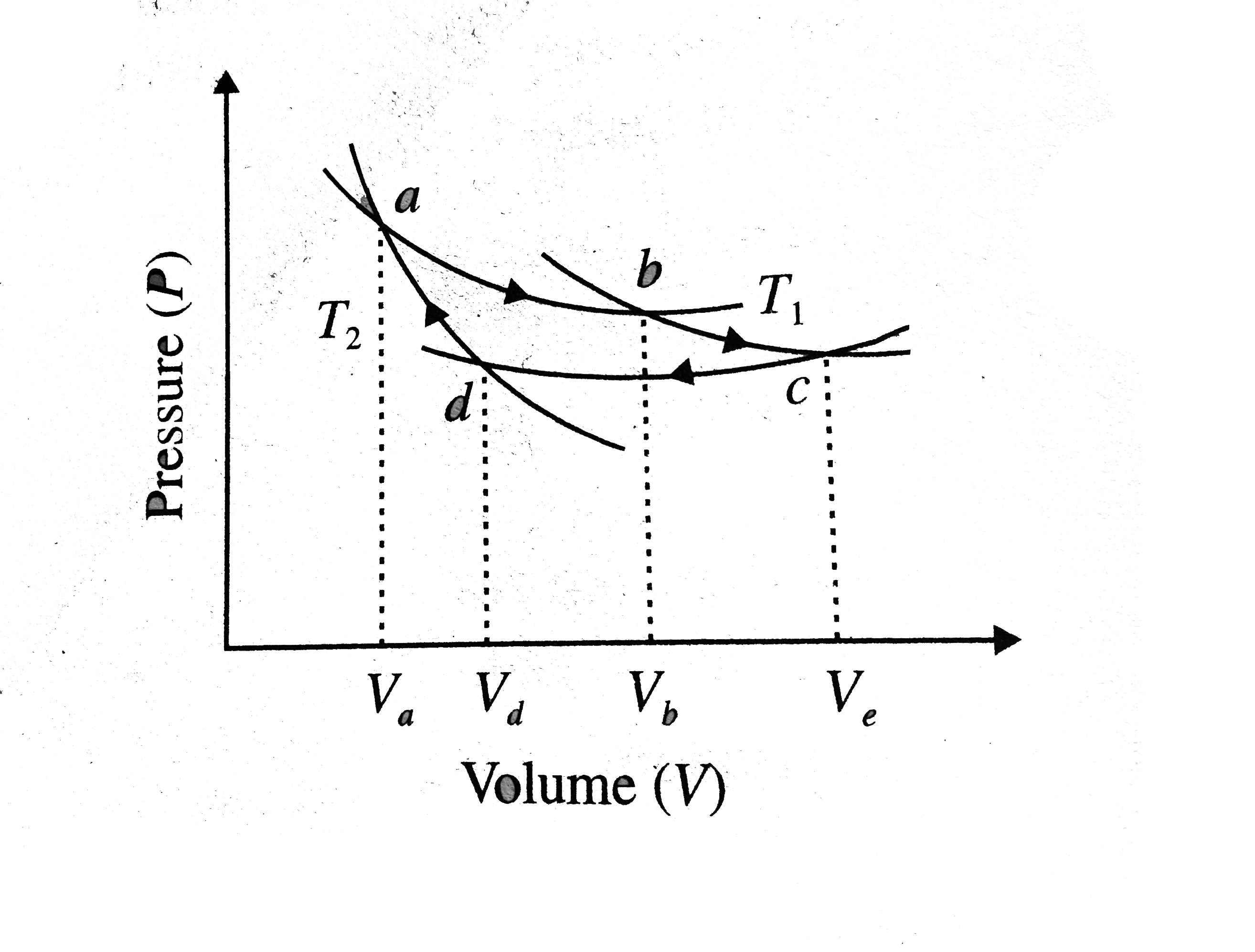 AT1=T2BT1>T2CVaVc=VbVdDVaVb=VcVd
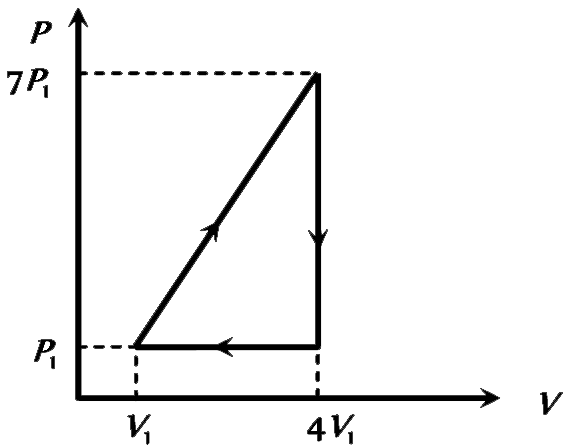
AT1=T2BT1>T2CVaVc=VbVdDVaVb=VcVdA monoatomic ideal gas undergoes a process ABC. The heat given to the gas is
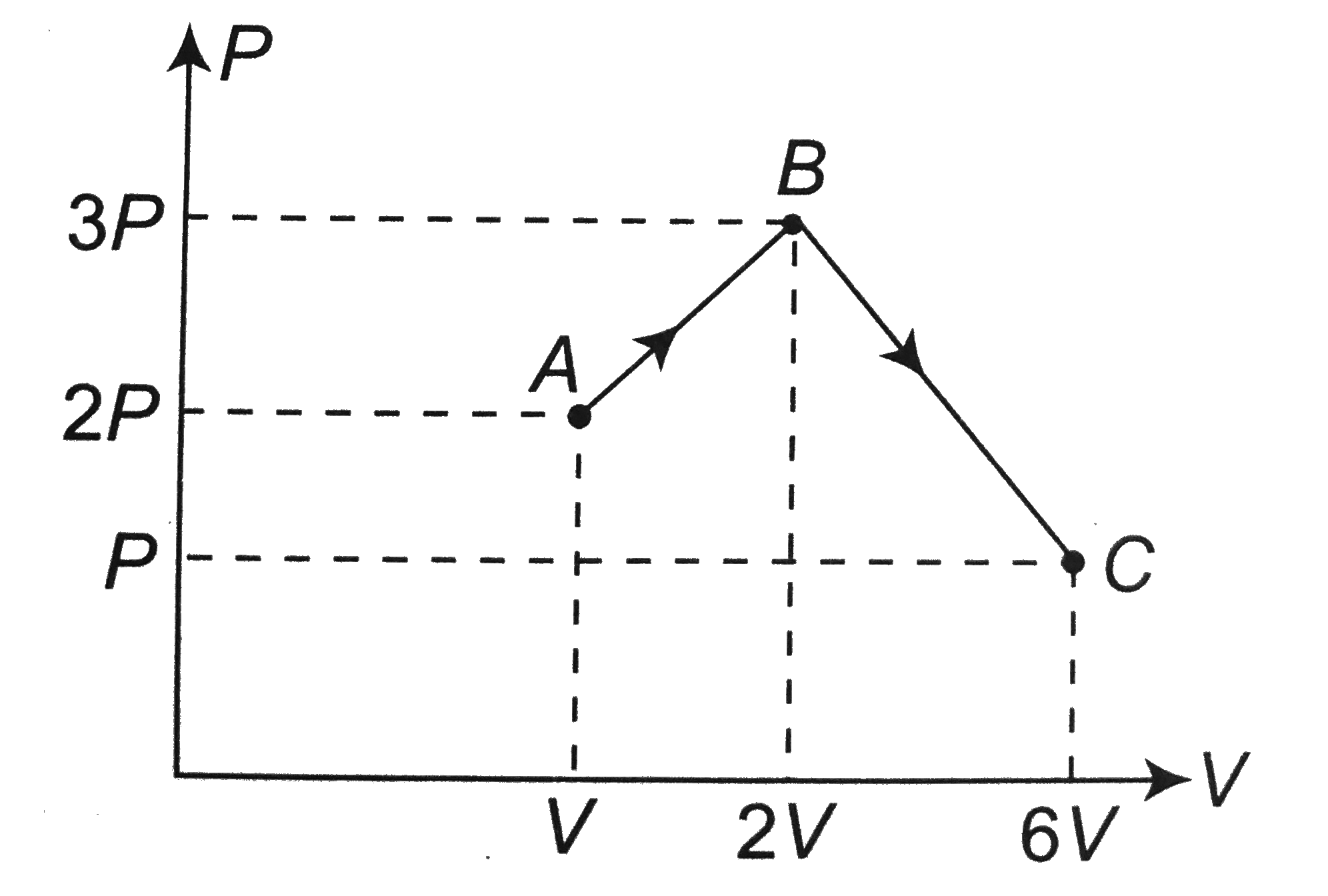 A7.5PVB12.5PVC16.5PVD20.5PV
A7.5PVB12.5PVC16.5PVD20.5PV
One mole of monoatomic gas is taken round the cyclic process. Heat absorbed by gas in process AB is
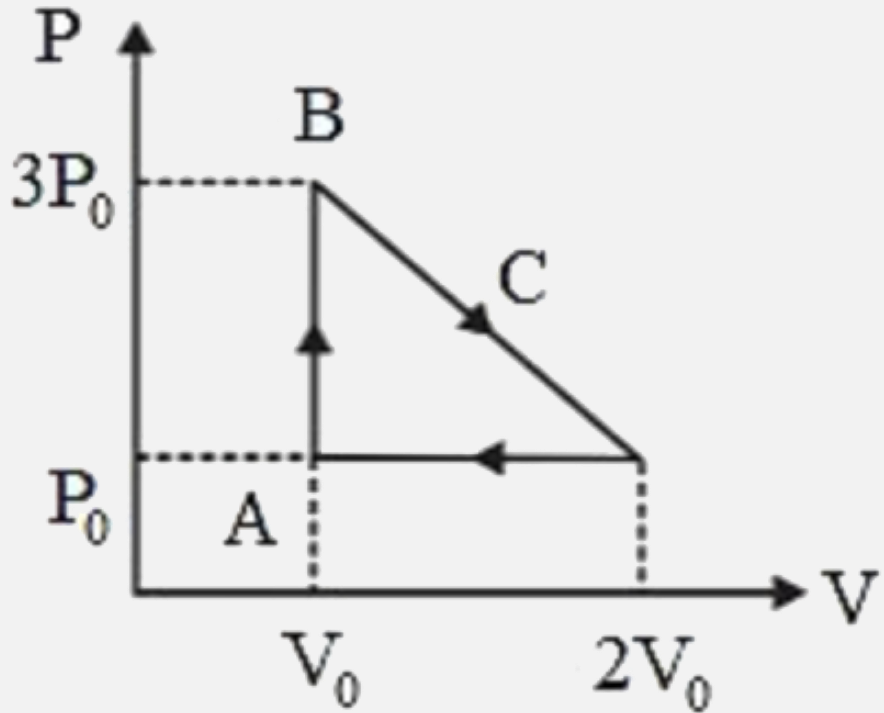
View SolutionAn ideal gas undergoes a cyclic process abcda which is shown by pressure-density curve,
View SolutionIn the cyclic process shown in the figure, the work done by the gas in one cycle is
View SolutionA sample of helium is undergoing a cyclic process ABCA as shown. In which of the following processes heat is a absorbed by the gas?
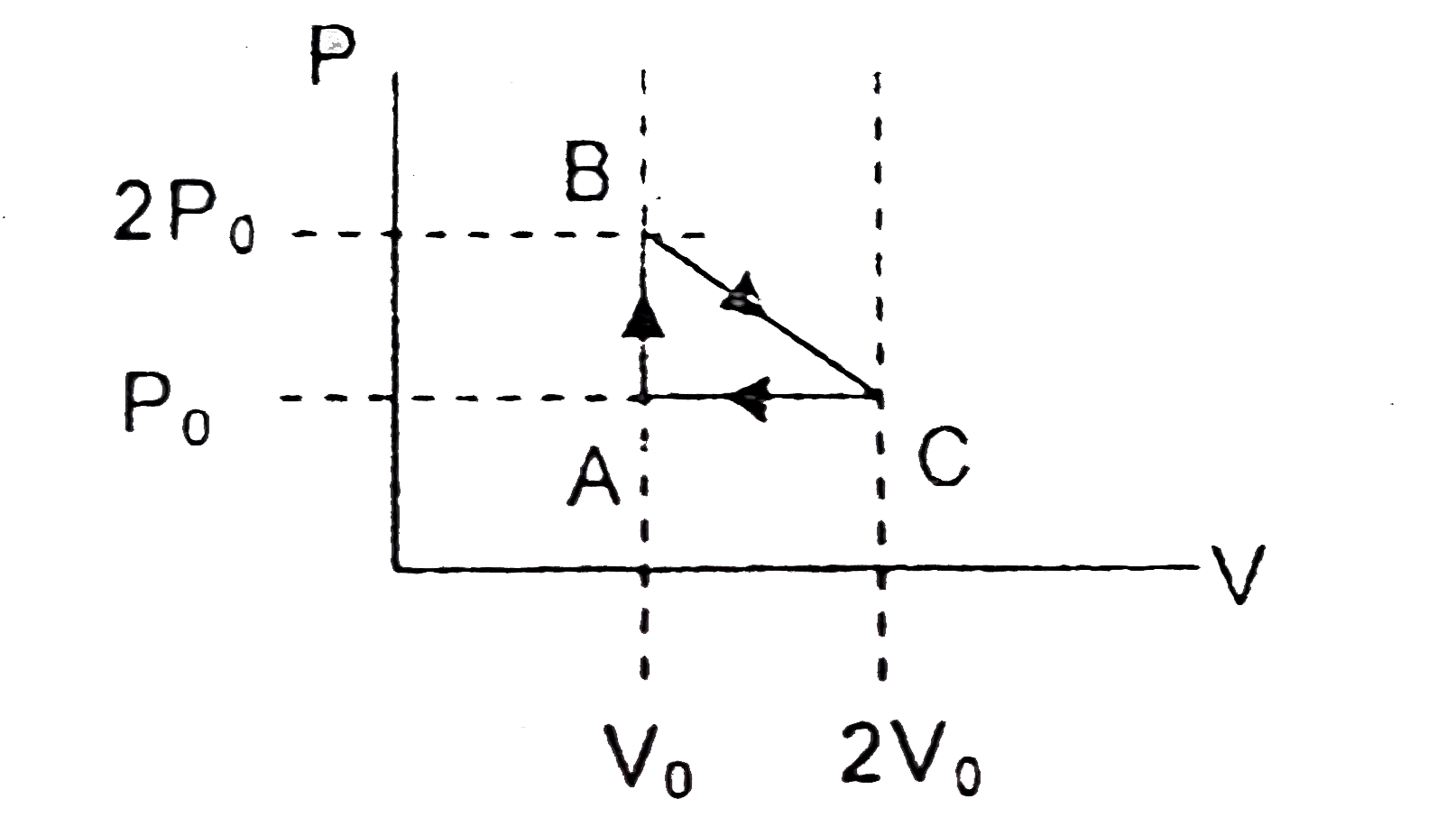
View SolutionIdeal gas is taken through process shown in figure :
View Solution
