Give the decreasing pH of aqueous solution of the following compounds :
a. NaCl , b. MgCl2 , c. AlCl3 , d. PCl5
Since anions are same, so higher the charge on the ions, more covalent and more acidic and hence less pH.
So, the decreasing order of pH is
1+NaCl>2+MgCl2>3+AlCl3>5+PCl5−−−−−−−−−−−−−−−−−−−−−→decreasing order of basic characterIncreasing order of acidic or[a.>b.>c.>d.]
Similar Questions
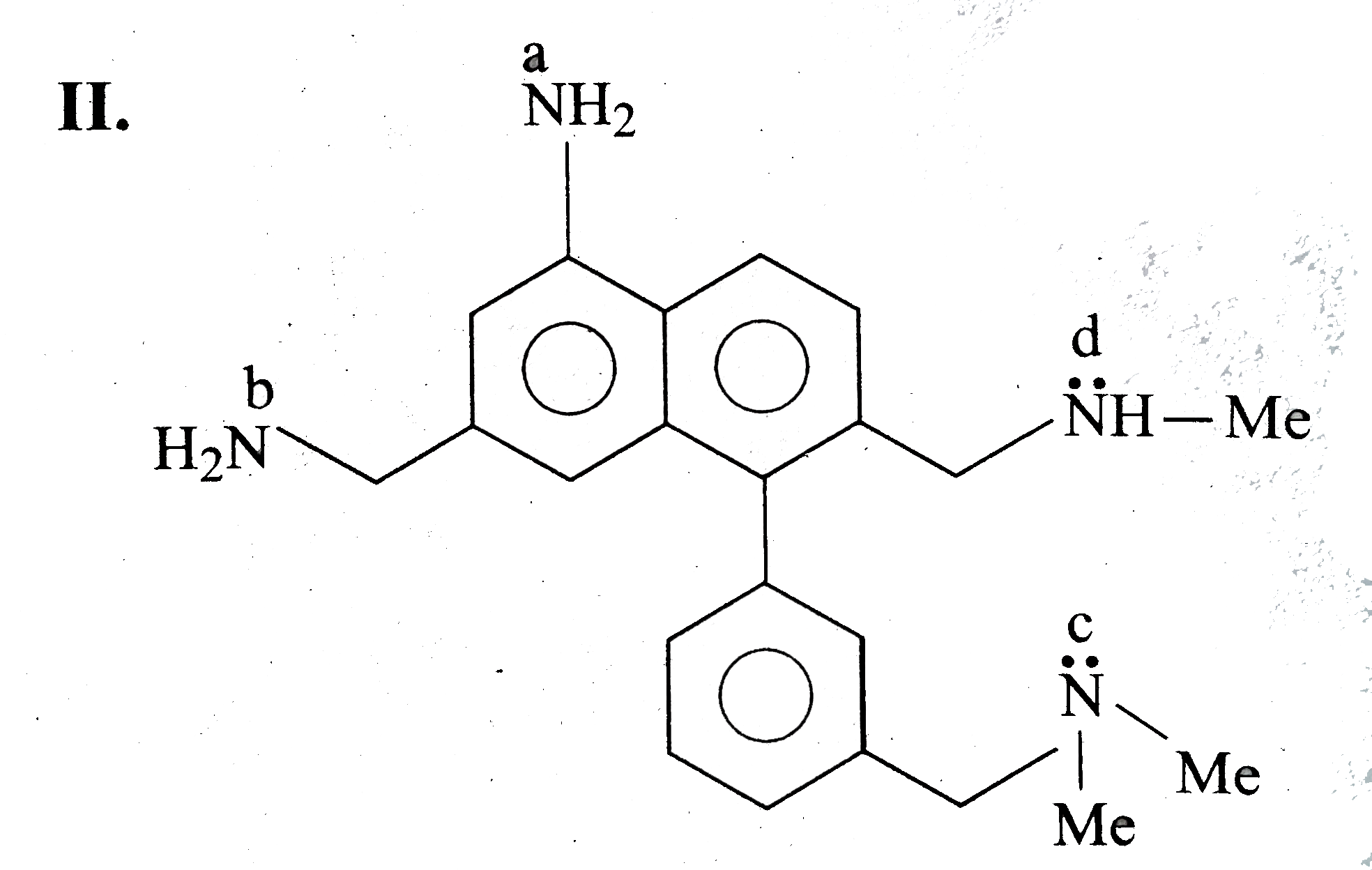
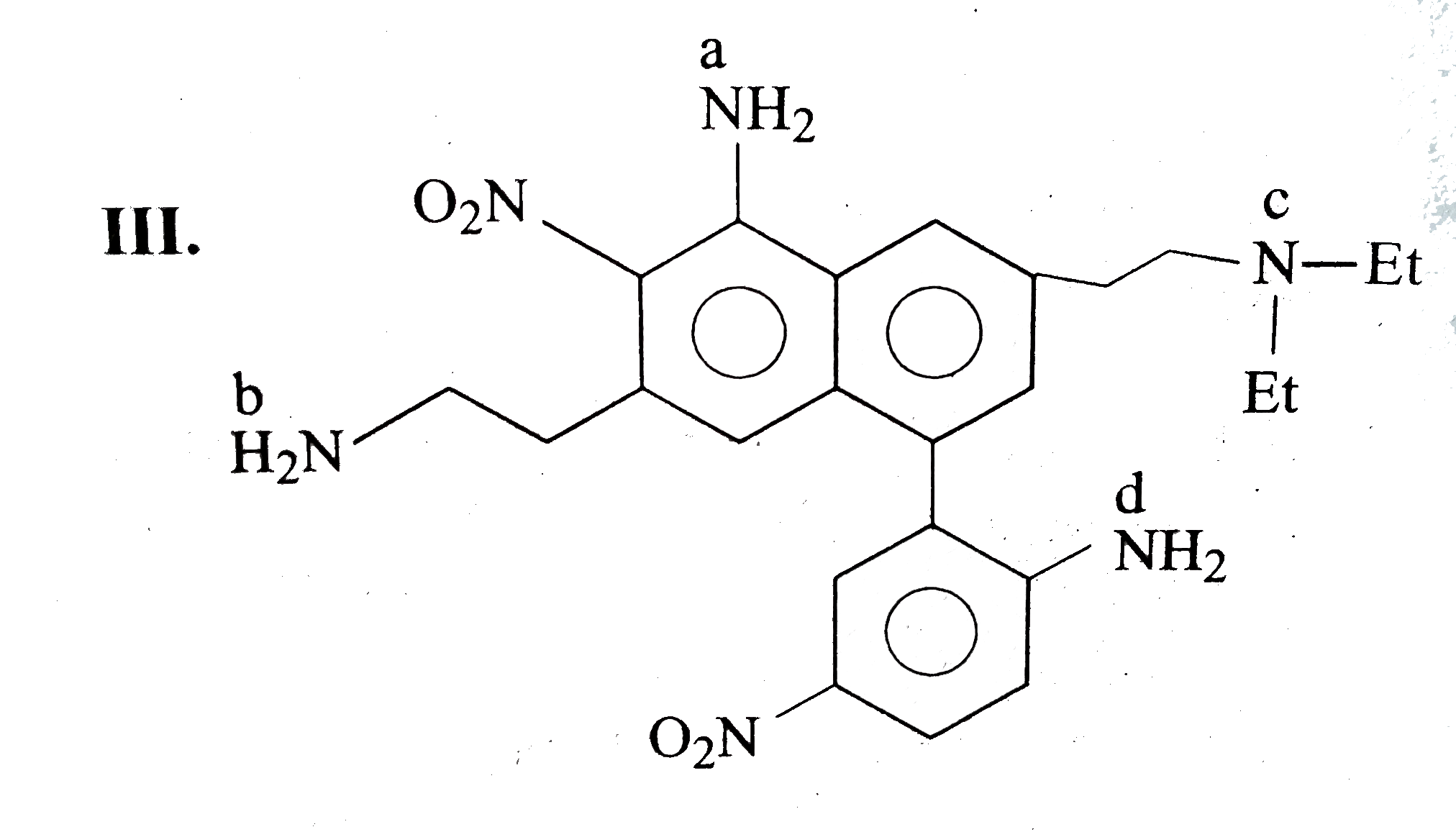
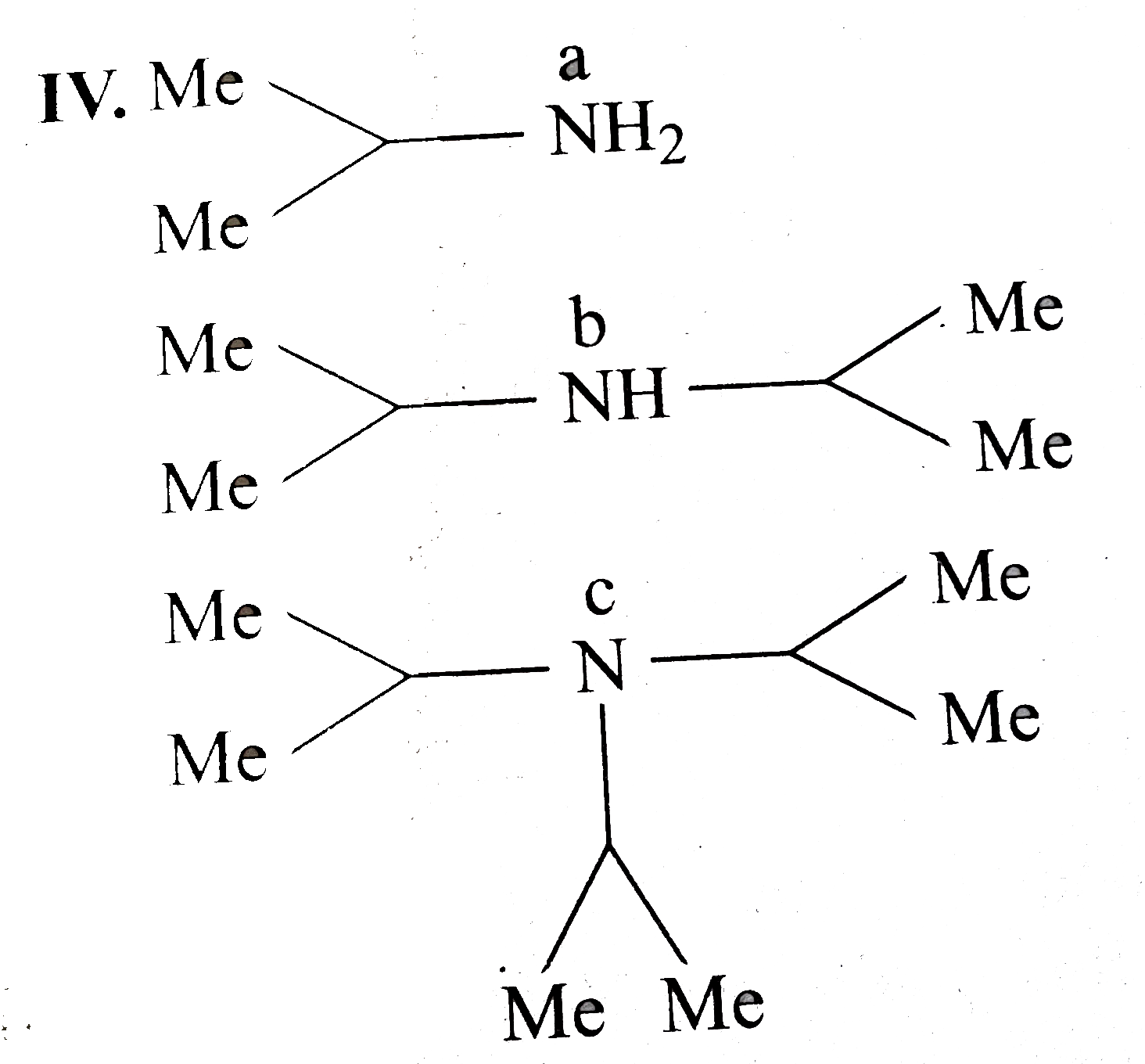
Give the decreasing order of basic character at a,b,c,d in the following compounds :
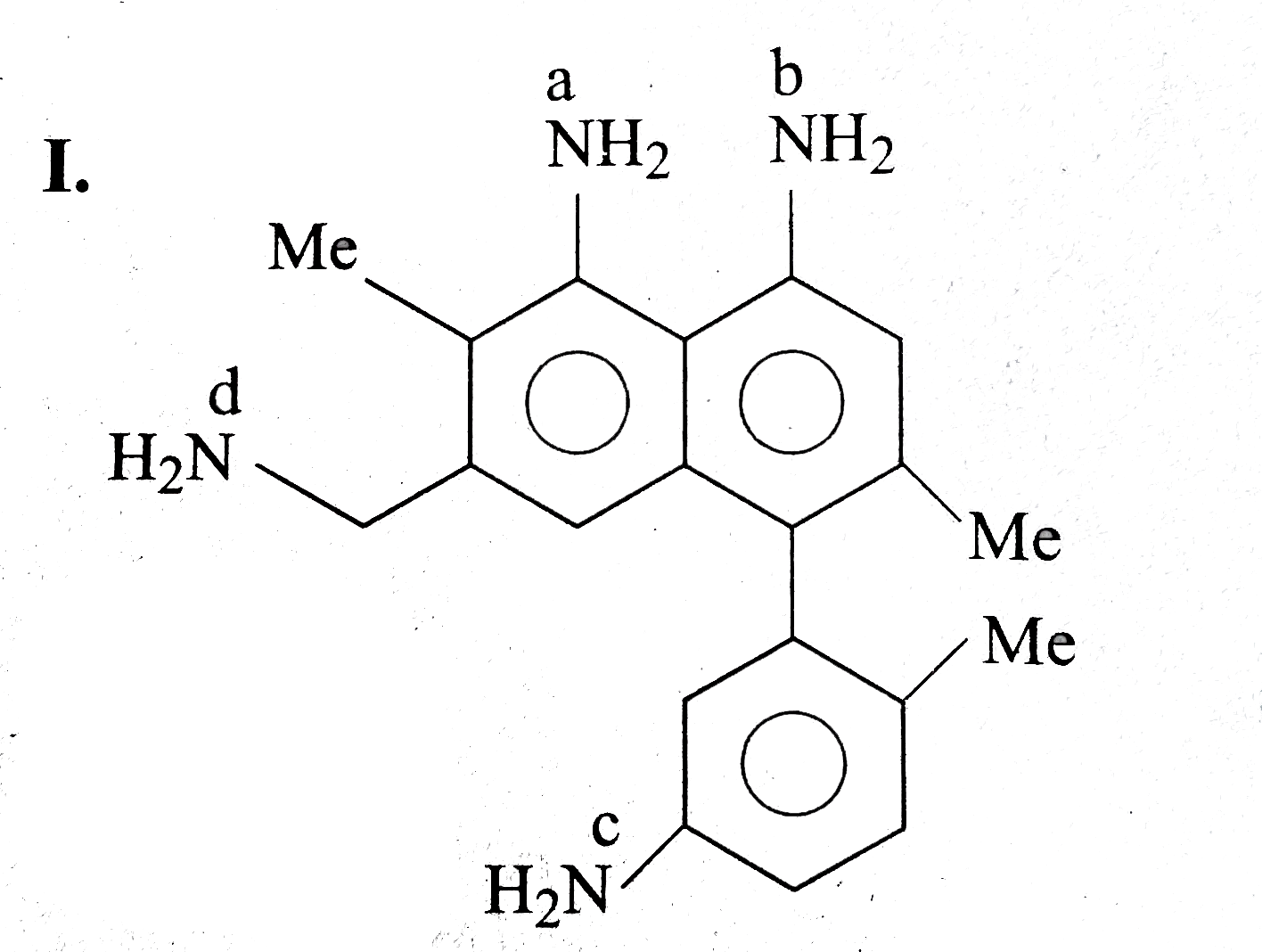 .
.
View SolutionGive the increasing order of melting points of the following compounds:
a. I. NaF , II. NaCl , III. NaBr, IV. NaI
b. I. CaI2 , II. CaBr2 , III. CaCl2 , IV. CaF2 , V. BeCl2
d. I. NaCl , II. MgCl2 , III. AlCl3
e. I. CCl4 , II. BCl3 , III. BeCl2 , IV. LiCl
View Solution
Knowledge Check
Aqueous solution of the following compound on electrolysis gives ethane
AAcetic acidBAcetamideCPotassium acetateDEthyl acetatepH of an aqueous solution of NaCl at 85∘C should be
A7B>7C<7D0The pH values of aqueous solutions of which of the following compounds does not change on dilution?
APhCOONH4BNH4CNCHCOONaDNH4CI
pH of an aqueous NaCl solution at 50∘C should be :
View SolutionWhich of the following will be the most hydrated salt ?
(1) NaCl (2) MgCl2 (3) AlCl3 (4) SiCl4
View SolutionWhich of the following will be the most hydrated salt ?
(1) NaCl (2) MgCl2 (3) AlCl3 (4) SiCl4
View SolutionWhich of the following will be the most hydrated salt ?
(1) NaCl (2) MgCl2 (3) AlCl3 (4) SiCl4
View SolutionThe correct order of increasing polarising power of the cations in the following AlCl3,MgCl3,NaCl is
View Solution
