Calculate the formal charge on each atoms in nitrite ion.
Video Solution
Text Solution
Lewis structure of NOΘ2 ion is
(a) FC on N-atom:
No. of valence e− on N-atom =5e−'s
Lone pair (lp) electrons on N-atom =1×2=2e−'s
No. of bonding e−'s=3×2=6e−'s
Hence FC on N-atom =5−2−12(6)=0
(b) FC on O-atom (marked 1)
No.of valence e−'s on O-atom 6e−'s
Lone pair (lp) electrons on O-atom =2×2=4e−'s
No. of bonds O-atom forms =2
No . of bonding e−'s=2×2=4e−'s
Hence FC on O-atom (marked 1) =6−4−12(4)=0
(c ) FC on O-atom (marked2) No of valence e− on O-atom =6e−'s
Lone pair (lp) electrons on O-atom =3×2=6
No of bonds O-atom forms =1
No of bonding e−'s=1×2=2e−'s
Hence FC on O-atom (marked2) =6−6−12(2)=−1
(d) Sum of FC's= Net charge of NOΘ2=−1
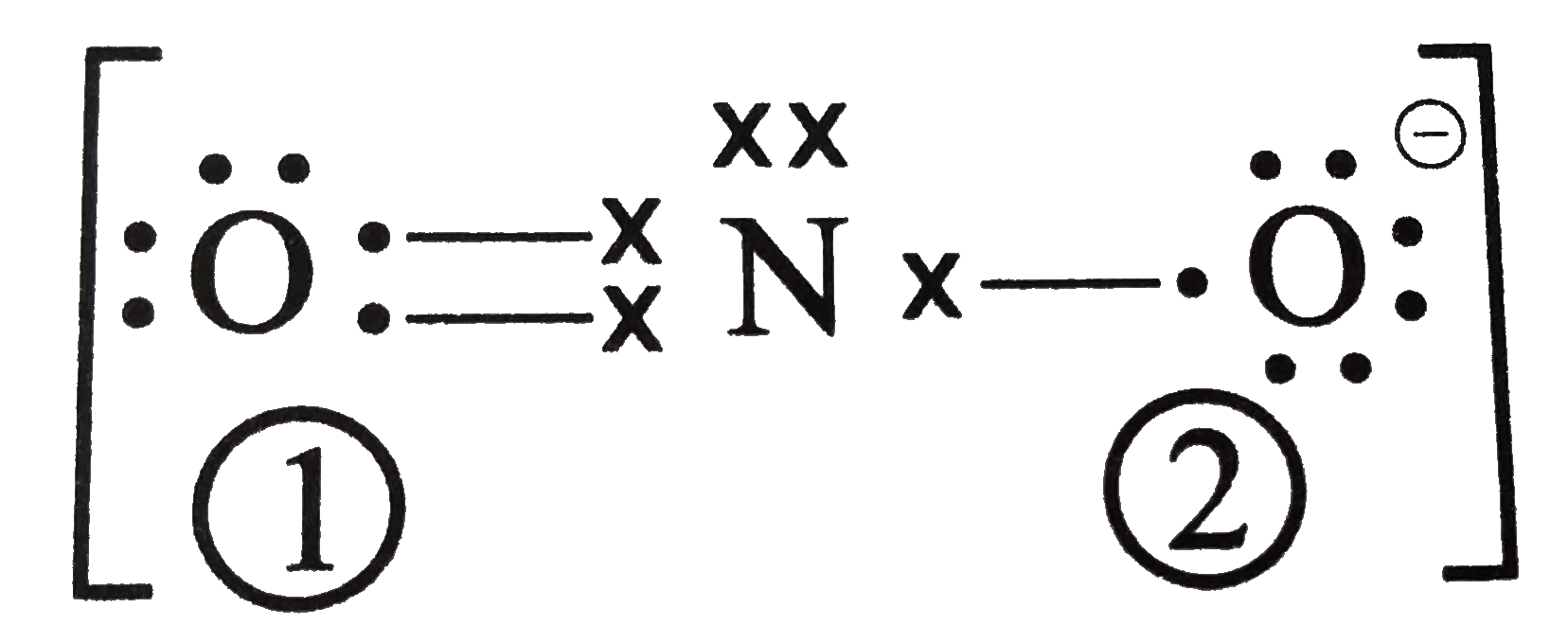 .
.
|
Similar Questions
Knowledge Check
Formal charge on two O atoms in
 A−1,+1B−1,0C0,+1D−1,−1
A−1,+1B−1,0C0,+1D−1,−1
Calcuate the formal charge on each atom in SO2−5 (per -oxosulphate ion) .
View SolutionCalculate the formal charge on atoms in carbonate (CO2−3) .
View SolutionCalculate the formal charge on Cl atom in HClO4.
View SolutionCalculate the formal charge on S atom in most stable structure of SO3
Hint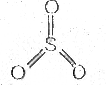
View SolutionCalculate the formal charge on each in the following electron-dot structure of SO2.
:⋅⋅O⋅⋅−⋅⋅S=⋅⋅O⋅⋅
View SolutionCalculate the formal charge on CI atom in HCIO4
Hint
View SolutionCalculate the formal charge and the oxiation state of Cr in chromate (CrO2−4)ion.

View Solution
