Give the decreasing order of the property mentioned against each of the following
C2H2,BF3,H2O,NH3,CH4 (bond angle)
(b) NH3,ΘNH2,⊕NH4 (bond angle)
( c) K−F,Li−CI,Na−I,Br−H,F−H,C−H (ionic character)
(d) NH3,PH3 (bond angle)
(e) NF3,NH3 (dipole mement)
(f) CH3CI,CH2CI2,CHCI3,CCI4 (dipole moment)
(g) MgO,CaO (hardness)
(h) HCI,HBr (ionic characacter)
(i) NO2,NO⊕2NOΘ2 (bond angle)
(j) CI2,O2,F2,N2 (bond strenght)
(k) O,N,F,CI,S (strength of H - bonging)
(I) N2,N⊕2,NΘ2,N2−2 (order of stability)
(m) O2+2,O2,O⊕2,OΘ2O2−2 (order of stability)
(n) N2,F2,O2,H2 (bond lenght) .
CH≡CH(180∘)>BF3(120∘)>CH4(109.5∘)[sp,linear][sp2,planar][sp3,TH]
..NH3(107∘)>H2..O:(104.5∘)>
⎡⎢⎣sp3,pyramidalRepusion betweenlp and bp⎤⎥⎦⎡⎢⎣sp3,V-shapeRepusion betweenlp and bp⎤⎥⎦
(b) ⊕NH4(109∘)>NH3(107∘)>:NH2(104∘)
[sp3,TH]⎡⎢⎣sp3,pyramidalRepusion betweenlp and bp⎤⎥⎦⎡⎢⎣sp3,V-shapeRepusion betweenlp and bp⎤⎥⎦
This is because all of them involve sp3 hybridisation The number of lp s of electrons on N-atom are 0.1 and 2 respectively Greater the number of lp s greater is the repulsions on the s and hence smaller is the angle
(c ) K−F>Na−I>Li−CI>F−H>Br−H>C−H
(Apply Fajans rule)
Refer to Section 1.17 (3) Point (v) NH3>PH3
(e) Refer to IIIuration 2.18 (a)
NH3>NF3
(f) Refer to IIIusation 2.19
CH3CI>CH2CI2>CHCI3>CCI4
(g) MgO>CaO (Hardness decreases down the 2nd group (⏐↓) due to decreases in lattice energy)
(h) HCI>HBr (Apply Fajans rule )
(i) Refer to section 1.17 (3) Point (v)
⊕NO2>NO2>N⊕O2.
⊕NO2 has no unshared electron and hence it is linear NO2 has no unshared electron while NOΘ2 has one unshared electron pair (i.e lone pairs of electrons)
(j) N≡N>O=O>CI−CI>F−F
Triple bond strenght gt doubel bond strenght gt single bond strenght
Bond stregth F−F is less than that of CI−CI due to repuslsion between the non-bonding e− s present on each very small F-atoms
(k) F>O>N>S>CI
Greater the EN and small size of the element it forms stroger H-bonding
(i) Refer to Table 2.19
Na2>N⊕2>NΘ2>n2−2
(m) Refer to Table 2.20
O2+2>O⊕2>O2>O⊕2>O2−2
(n) F2(F−F)>O2(O=O)>N2(N≡N)>H2(H−H)
[Bondxlenght (pm)]144>121>109>74
Bond lenght of single bond gt double bond gt triple bond
Since the H-atom is very small size so bond lenght of single bond between H and H is the smallest .
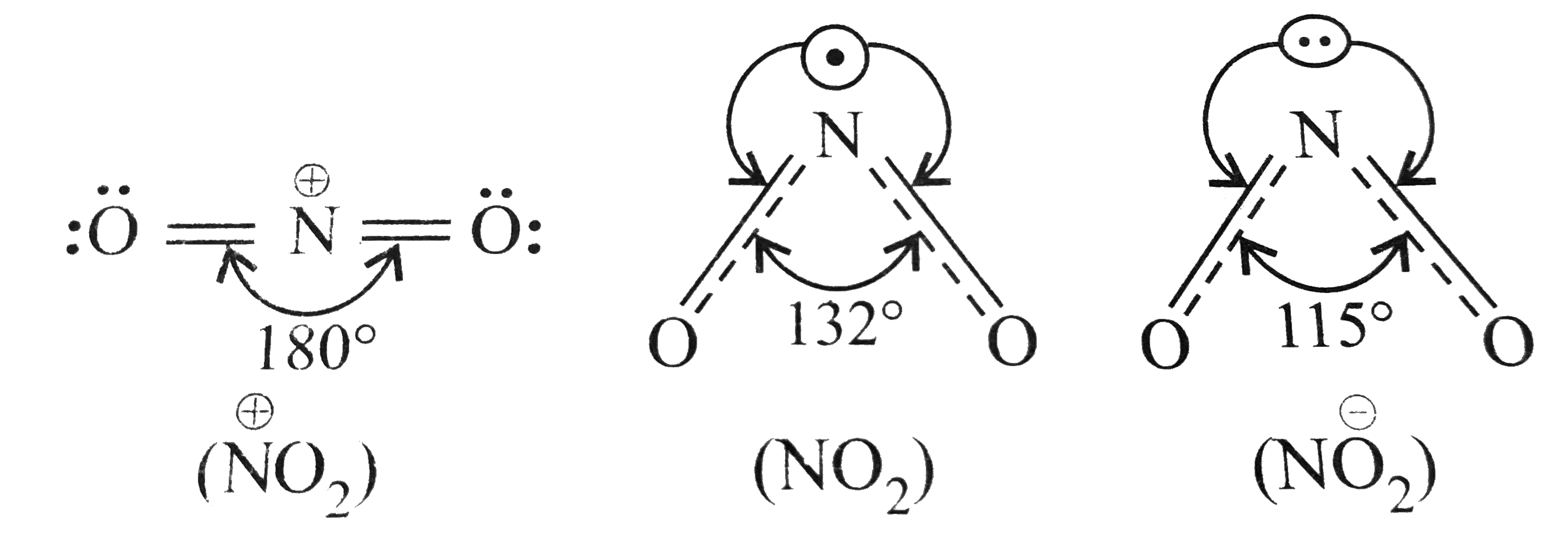 .
.
Similar Questions
Classify the following into electrophilies and nucleophiles: (a) H^(+) , (b) NH_(3) (c) AlCl_(3) , (d) NO_(2)^(+) (e) CN^(-) , (f) H_(2)O (g) ROH , (h) H_(2)O (i) Carbocation.
View SolutionUsing IUPAC norms write the systematic names of the following : (a) [Co(NH_(3))_(6)]CI_(3) (b) [Co(NH_(3))_(4)CI(NO_(2))]CI (c) [Ni(NH_(3))_(6)]CL_(2) (d) [Pt(NH_(3))_(2)CI(NH_(2)CH_(3))]CI (e) [Mn(H_(2)O)_(6)]^(2+) (f) [NiCl_(4)]^(2-) (g) [Co(en)_(3)]^(3+) (h) [Ti(H_(2)O)_(6)]^(3+) (i) Ni(CO)_(4) .
View SolutionArrange the following in order of decreasing bond angles (i)CH_(4), NH_(3), H_(2)O, BF_(3), C_(2) H_(2) " "(ii) NH_(3), NH_(2)^(-), NH_(4) ^(+)
View SolutionArrange the following in the decreasing order of Bond angle CH_(4) , H_(2)O, NH_(3)
View SolutionArrange the following in order of decreasing bond angles (i) CH_(4), NH_(3), H_(2)O, BF_(3), C_(2)H_(2)" "(ii) NH_(3), NH_(2)^(-), NH_(4)^(+)
View SolutionArrange the following sets of molecules in the decreasing order of bond angle :
(i) SF6,CCl4,H2O,NH3
(ii) CH4,NH3,H2O,BF3
(iii) AlCl3,H2S,BeH2,H2O
View SolutionThe correct order of bond angles among the compounds CH4, NH3 and H2O is
(A) H2O < NH3 < CH4
(B) NH3 < H2O < CH4
(C) NH3 < CH4 < H2O
(D) CH4 < H2O < NH3
View SolutionThe decreasing order of alkaline strength is: (I) CH_(3)NH_(2) (II) C_(6)H_(5)CH_(2)NH_(2) (III) p-CH_(3)O-C_(6)H_(4)-NH_(2) (IV) m-CH_(3)O -C_(6)H_(4) - NH_(2)
View Solution

