Peroxo bond (−O−O−) is present in
A
Na2O2
B
CrO5
C
PbO2
D
SrO2
Video Solution
Text Solution
The correct Answer is:A, B, D
Peroxo bond is O2−2
(a) Na2O2→2Na⊕+O2−2
(c ) PbO2 is oxide
(d) SrO2→Sr+2+O2−2
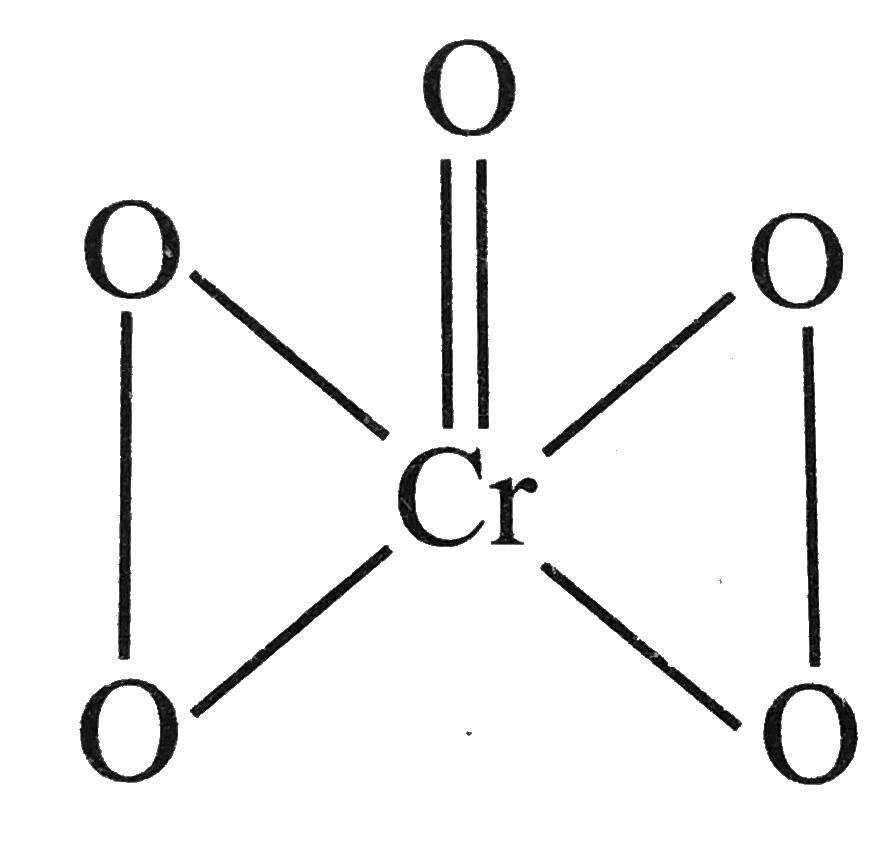 .
.
|
Similar Questions
Knowledge Check
P-O-P bond is present in
AH4P2O6BH4P2O5Cboth (A) and BDNone of theseP-O-P bond is present in
AH4P2O6BH4P2O5CBoth (A) and (B)DNone of theseO- O ' peroxy bond is present in
AH2SO5BHNO4CH2S2O8DAll of these
Shortest C-O bond is present in
View SolutionStrongest C-O bond is present in
View SolutionThe number of P-O-P bonds present in P4O6andP4O10 are respectively
View SolutionStatement 1: o-nitrophenol has higher boilling point than p-nitrophenol.
Statement 2: Intermolecular hydrogen bonding is present in p-nitrophenol and intrmolecular hydrogen bonding in o-nitrophenol.
View SolutionThe acid in which O-O bonding is present in :
View Solution

