In which of the following molecules all the atoms lie in one plane ? .
CH4
BF3
PF5
NH3
The correct Answer is:B
To determine which of the given molecules has all its atoms lying in one plane, we will analyze each molecule based on its hybridization and molecular geometry.
1. Analyze CH4 (Methane):
- The carbon atom in CH4 is sp³ hybridized.
- In sp³ hybridization, the geometry is tetrahedral.
- This means that the four hydrogen atoms are not in the same plane; they are arranged in three-dimensional space.
- Conclusion: CH4 does not have all atoms in one plane.
2. Analyze BF3 (Boron Trifluoride):
- The boron atom in BF3 is sp² hybridized.
- In sp² hybridization, the geometry is trigonal planar.
- This means that the three fluorine atoms are arranged in the same plane with bond angles of 120 degrees.
- Conclusion: BF3 has all atoms in one plane.
3. Analyze PF5 (Phosphorus Pentafluoride):
- The phosphorus atom in PF5 is sp³d hybridized.
- The geometry is trigonal bipyramidal.
- In this structure, there are two fluorine atoms positioned above and below the plane of the three fluorine atoms, which lie in a plane.
- Conclusion: PF5 does not have all atoms in one plane.
4. Analyze NH3 (Ammonia):
- The nitrogen atom in NH3 is sp³ hybridized.
- The geometry is also tetrahedral.
- NH3 has one lone pair and three hydrogen atoms, which means that the hydrogen atoms are not all in the same plane.
- Conclusion: NH3 does not have all atoms in one plane.
Final Answer:
The molecule in which all the atoms lie in one plane is BF3.
---
- The carbon atom in CH4 is sp³ hybridized.
- In sp³ hybridization, the geometry is tetrahedral.
- This means that the four hydrogen atoms are not in the same plane; they are arranged in three-dimensional space.
- Conclusion: CH4 does not have all atoms in one plane.
- The boron atom in BF3 is sp² hybridized.
- In sp² hybridization, the geometry is trigonal planar.
- This means that the three fluorine atoms are arranged in the same plane with bond angles of 120 degrees.
- Conclusion: BF3 has all atoms in one plane.
- The phosphorus atom in PF5 is sp³d hybridized.
- The geometry is trigonal bipyramidal.
- In this structure, there are two fluorine atoms positioned above and below the plane of the three fluorine atoms, which lie in a plane.
- Conclusion: PF5 does not have all atoms in one plane.
- The nitrogen atom in NH3 is sp³ hybridized.
- The geometry is also tetrahedral.
- NH3 has one lone pair and three hydrogen atoms, which means that the hydrogen atoms are not all in the same plane.
- Conclusion: NH3 does not have all atoms in one plane.
The molecule in which all the atoms lie in one plane is BF3.
Similar Questions
Knowledge Check
In which of the following molecules, all atoms are not coplanar ?
A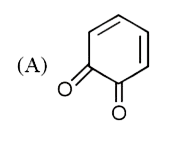 B
B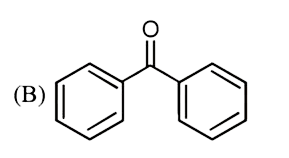 C
C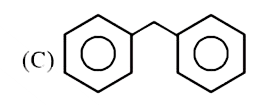 D
D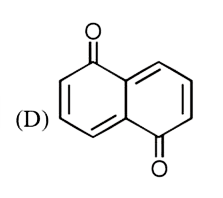
In which of the following moleucles, all atoms are coplanar?
A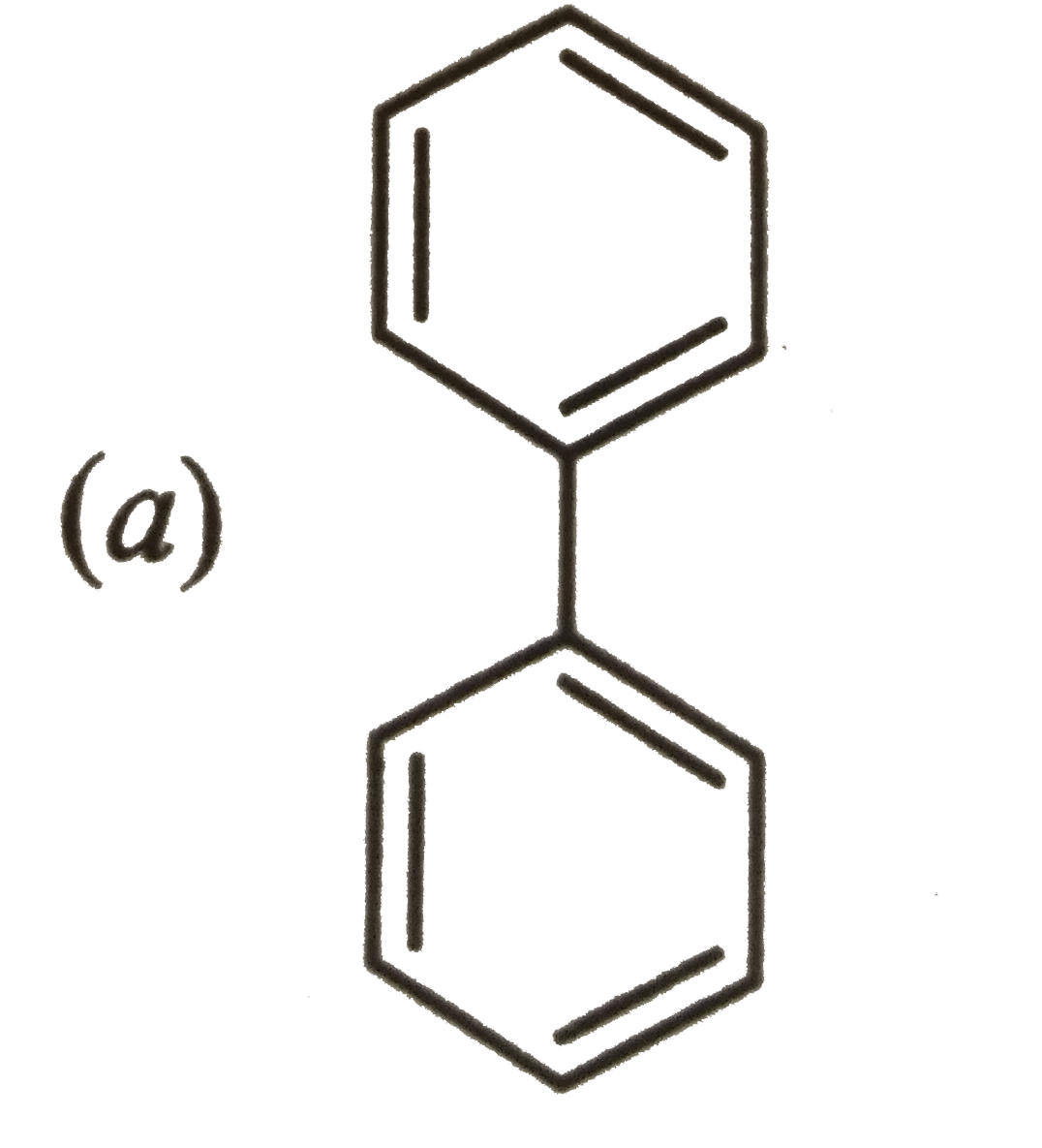 B
B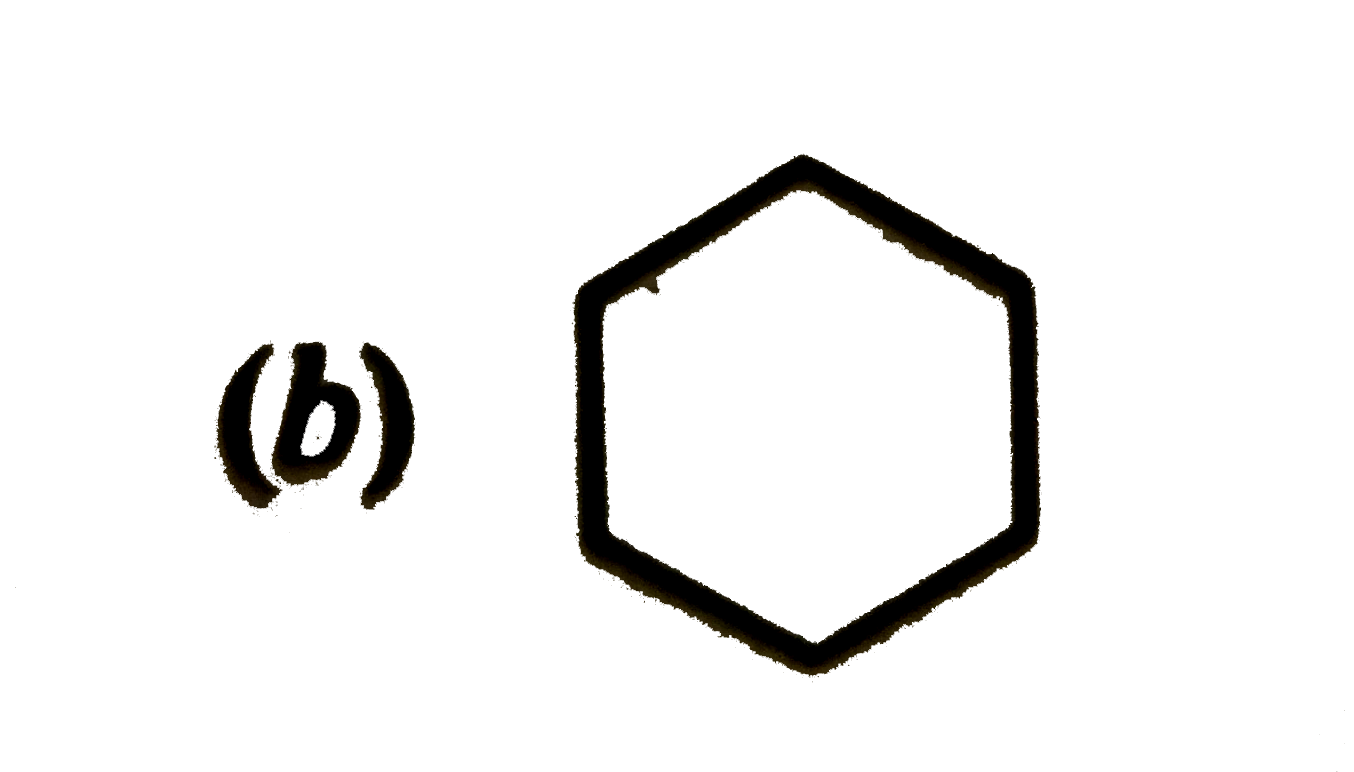 C
C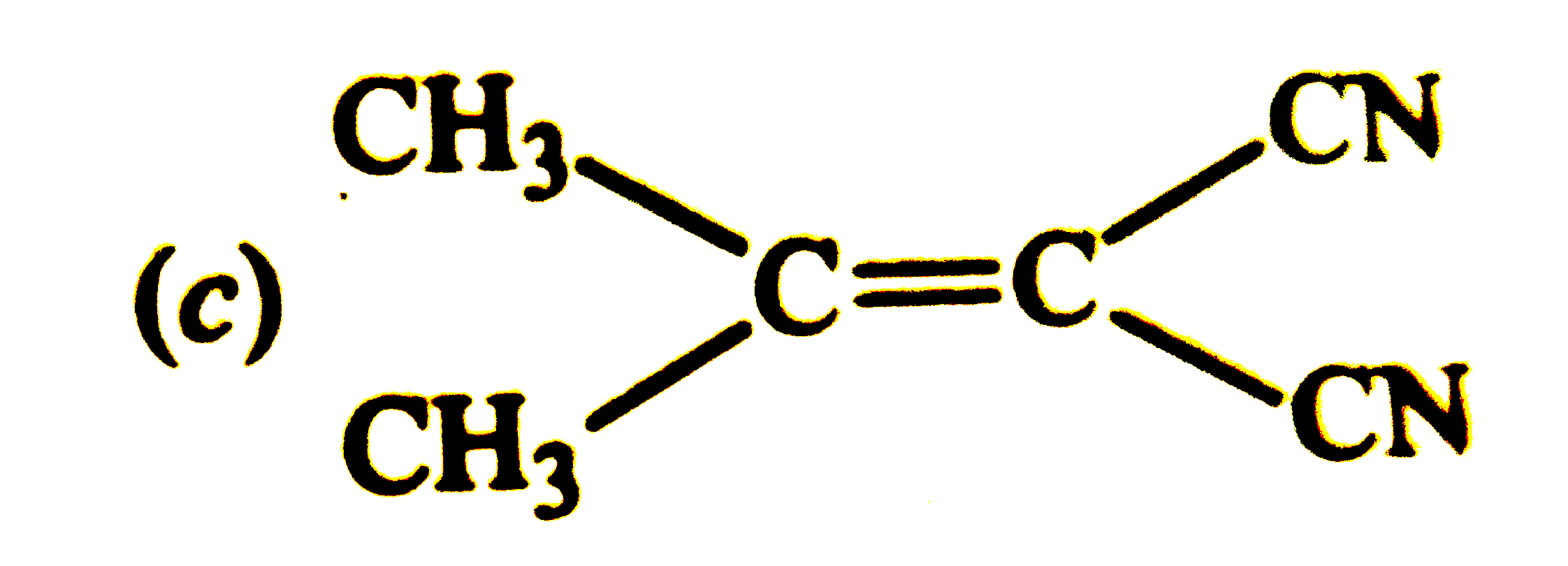 D
D
In which of the following molecules, all atoms are coplanar ?
A B
B C
C D
D
In which of the following molecules all the bonds are not equal:-
View SolutionIn how many of the following molecules, all atoms are in same plane?
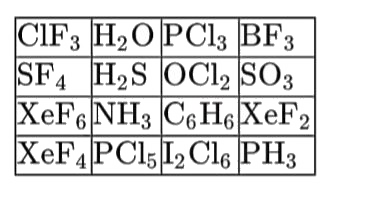
View SolutionIn which of the following molecule/ion all the bonds are not equal?
View SolutionIn which of the following molecules are all the bonds not equal ?
View SolutionIn which of the following molecules are all the bonds not equal ?
View Solution

