The number of correct options is
(a) 1Θ>BrΘ>CIΘ>FΘ (polarisability)
(b) Li⊕>Na⊕>K⊕>Rb⊕ (polarisation power)
(c ) H2O>H2S>H2Se>H2 Te (order of b.pt)
(d) HΘ2<H⊕2 (order of stability) .
Video Solution
Text Solution
The correct Answer is:3
(a),(b) and (d) correct options
(a) IΘ>BrΘ>CIΘ>FΘ (Polarisability)
According to Fajans' rule small cation is more polarisable
(c ) Wrong and
Boiling point order is
Both HΘ2 have same bond order of 0.5 H⊕2 is more stable than HΘ2, since H⊕2 contains one e− in antibonding orbitals .
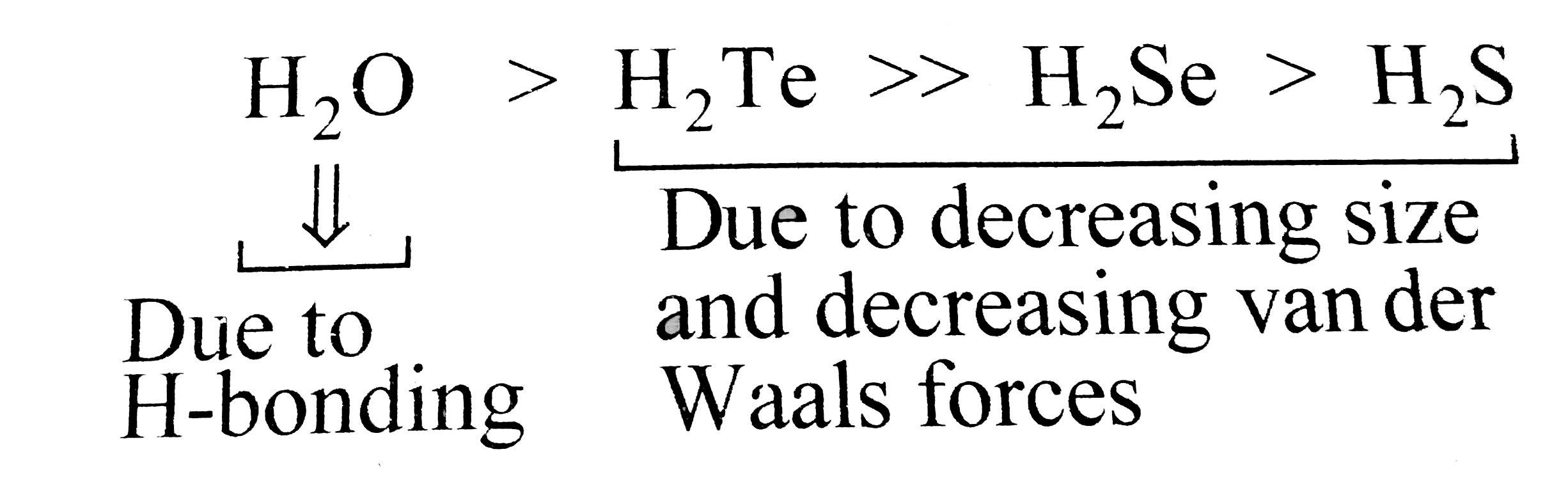
|
Similar Questions
Knowledge Check
In the reaction I2+2OHΘ→IΘ+IOΘ+H2OI2 is
AOxidisedBReducedCOxidised and reducedDForms complexThe boiling points of H2O,H2S,H2Se and H2Te are in the following order
AH2O>H2S>H2Se>H2TeBH2O>H2Te>H2Se>H2SCH2Te>H2Se>H2S>H2ODH2S>H2O>H2Te>H2SeOrder H3PO4>H2SO4>HNO3>HCl is correct for :-
AAcidic natureBBasic strengthCViscosityDOxidation state
The number of trends, which are true with respect to the properties of hydrides of oxygen family:
1) Bond angle:
H2O>H2S>H2Se>H2Te
2) Volatility :
H2O<H2S>H2Se>H2Te
3) Thermal stability:
H2O>H2S>H2Se>H2Te
4) Acidic strength :
H2O>H2S>H2Se>H2Te
View SolutionArrange the following molecules in the increasing order of bond angle.
H2OIH2SIIH2SeIIIH2TeIV
View SolutionConsider the following orders:
(1) H2SO4>H2SO3:boiling point
(2) H2O>HF: Extent of H-bond
(3) H2O<H2O2: Strength off H-bond.
View SolutionWhich one of the following is correct order?
(A) T2>D2>H2 (order of BP)
(B) T2>D2>H2 (order of BE)
(C) T2=D2=H2 (order of BL)
(D) T2<D2<H2 (order of reactivity with Cl2)
View SolutionIn the reaction: 3Br2+6OHΘ→5BrΘ+BrOΘ3+3H2O,Br2 is
View Solution

