Which of the following compounds has a zero dipole momnet ? .
A
1,1-Dichloroethylene
B
cis-1,2Dicloroehtylene
C
trans-1,2-Dichloroethylene
D
None of these
Video Solution
Text Solution
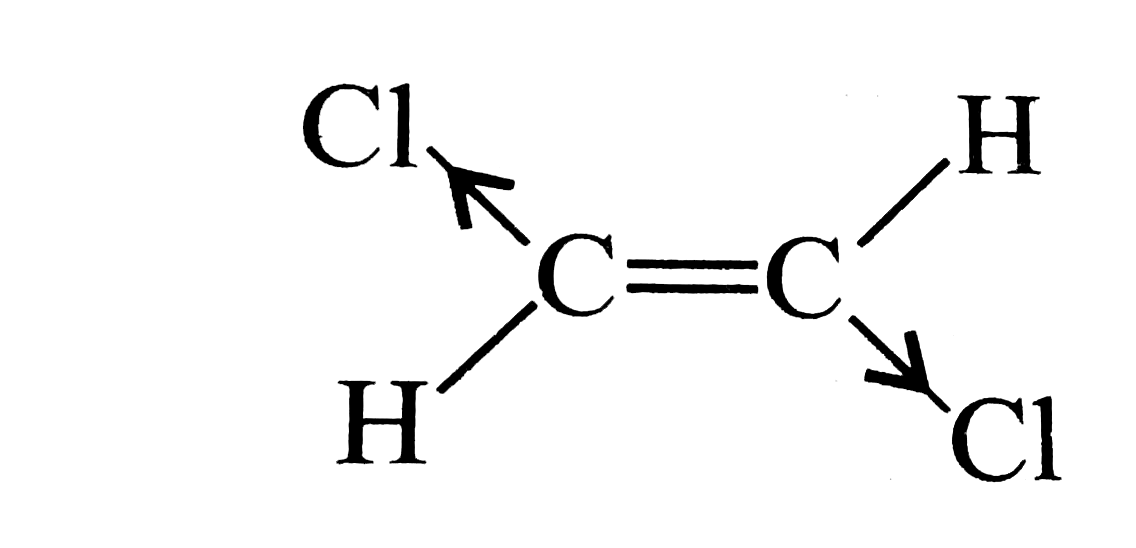
The correct Answer is:C
in trans 1,2 dichloroethylene net dipole momnet is zero because the two dipole moment vectors are equal and opposite and they cancel each other as shown above
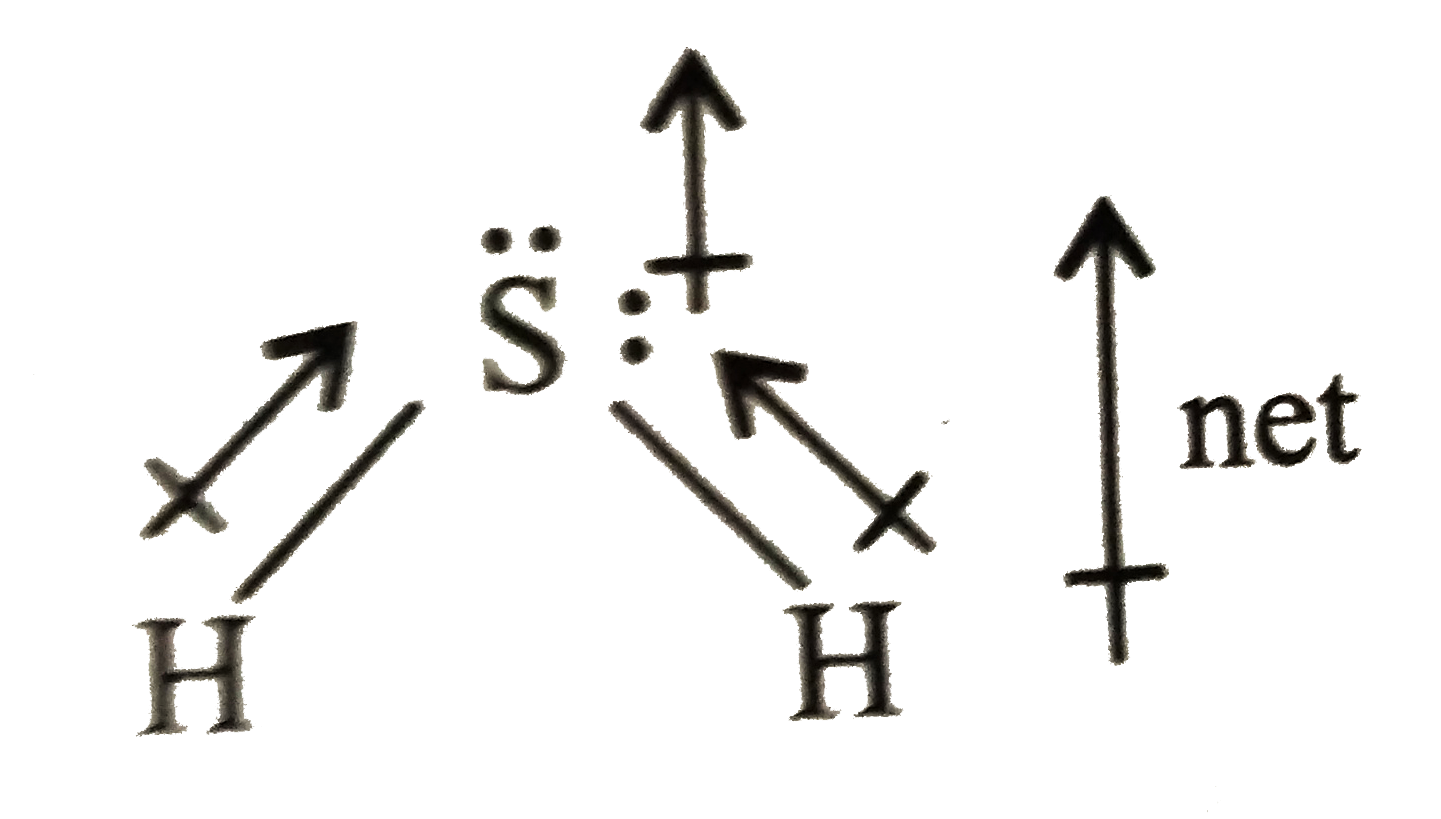 .
.
|
Similar Questions
Which of the following compound has zero dipole moment?
View SolutionElectric Dipole moment is a vector quantify . If a compound contain more than one polar bond then , the net electric dipole moment is equal to vector sum of individual dipole moment .
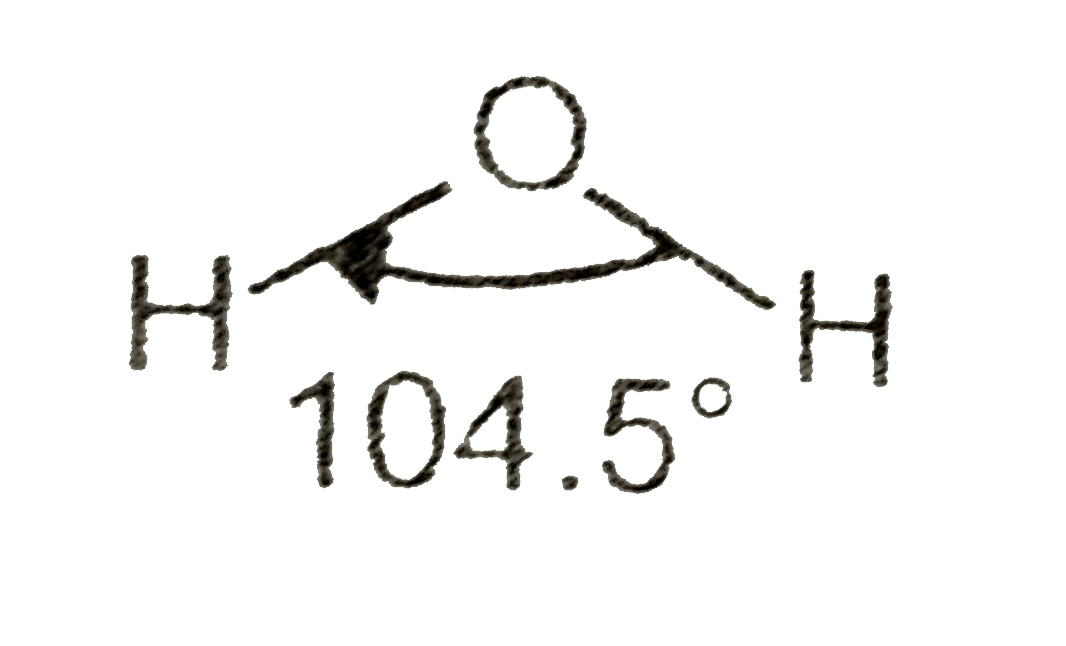
e.g., In H2O
μ=1.84D
Which of the following compounds have zero dipole moment ?
View Solution
Knowledge Check
Which of the following compounds has a zero dipole moment ?
A B
B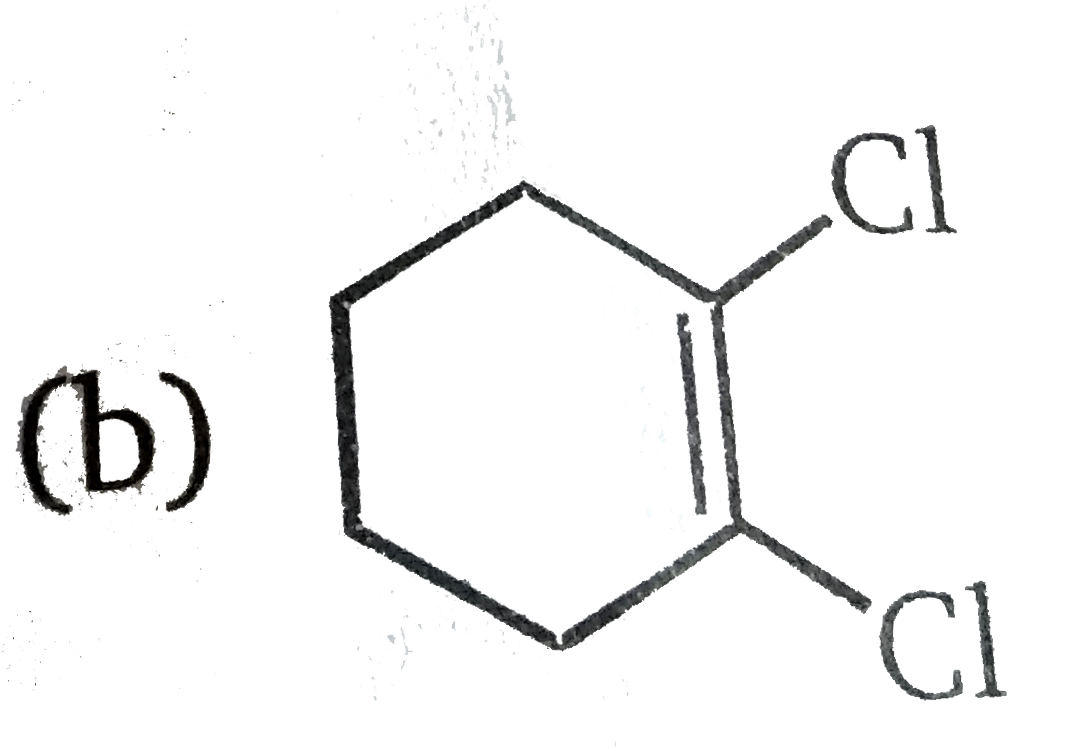 C
C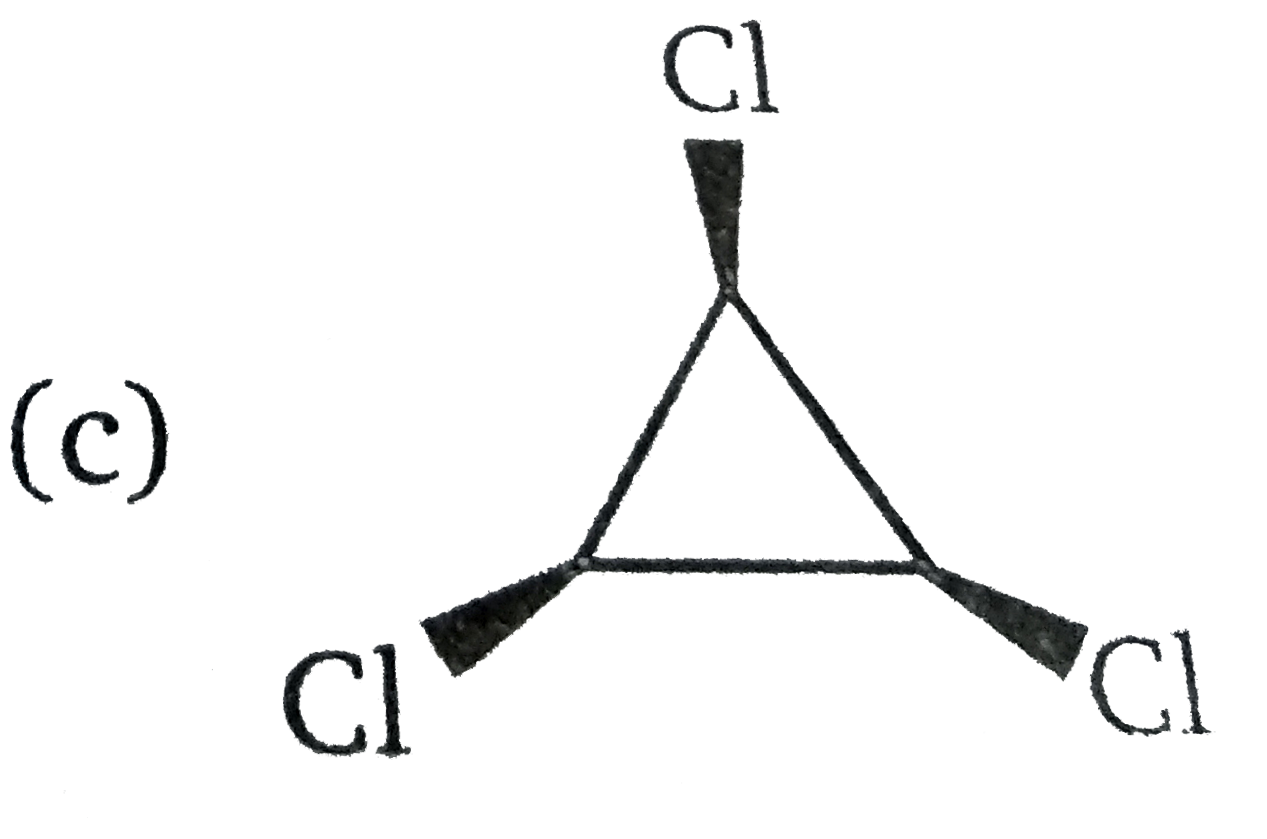 D
D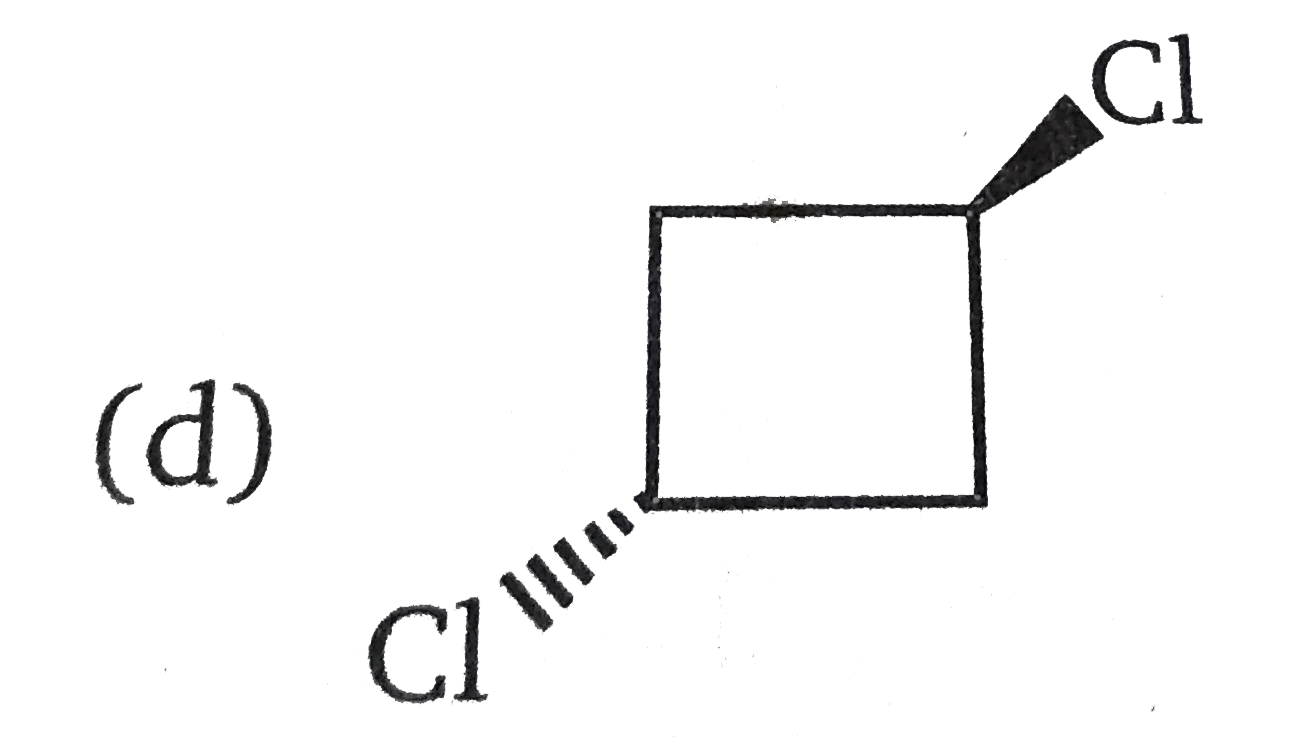
Which of the following compound has zero dipole moment?
A1,1-Dichloro ethyleneBCis-1,2-dichoroethyleneCTrans-1,2-dichoroethyleneDBoth 2 and 3Which of the following compounds have zero dipole moment ?
ABF3BSnCl2CH2ODNH3
Which of the following compounds has a non-zero dipole moment/
View SolutionWhich of the following compound has non-zero dipole moment
View SolutionWhich of the following compounds have non zero dipole moment.
View SolutionWhich one of the following compounds has non zero dipole moment ?
View Solutionwhich of the following compound has zero dipole moment in one of the stable conformations?
View Solution
