Using the VSEPR theory, identify the type of hybisation and draw the structure of OF2 What are the oxidation states of O and F ? .
Video Solution
Text Solution
In OF2 there are two lone pairs on oxygen atom three lone pairs on each of the F atoms Also there are two bond pairs of electrons In OF2 oxidation state of oxygen is +2 while the oxidation state of fluorine is -1 it is known as oxygen difluoride and not as oxide becasue ENofF>O
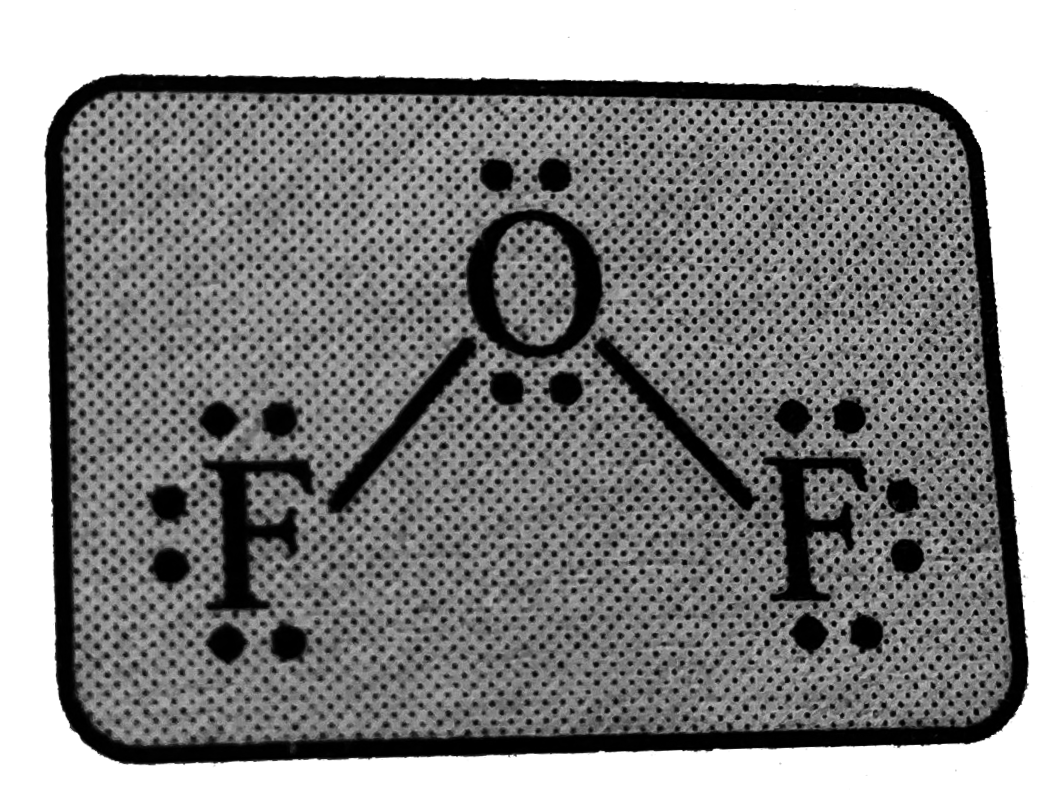
 .
.
|
Similar Questions
Knowledge Check
The oxidation state of 'O' in O2 is
A−2B+1C0D+2The oxidation state of O in Na2O2 is
A+2B-2C-1D+1The oxidation state of O-atom is +2 in
AOF2BO2F2CH2ODH2O2
