Hydrogen forms three types of bond in its compounds. Describe each type of bonding using suitable examples.
Video Solution
Text Solution
a. Ionic bond: The binary hydrides of alkali metals (such as LiH,NaH,KH etc.) form ionic nond. they on electrolysis give hydride ion Hɵ.
NaHs+H2Oaq→H2(g)+NaOHaq
Na⊕+Hɵ→NaH
b. Metallic bond: d− and f− block elements (metals) form metallic bond, Examples: ScH2,YH2,LaH3 etc.
c. Covalent bond: With p-block elements hydrogen forms covalent bond for expample
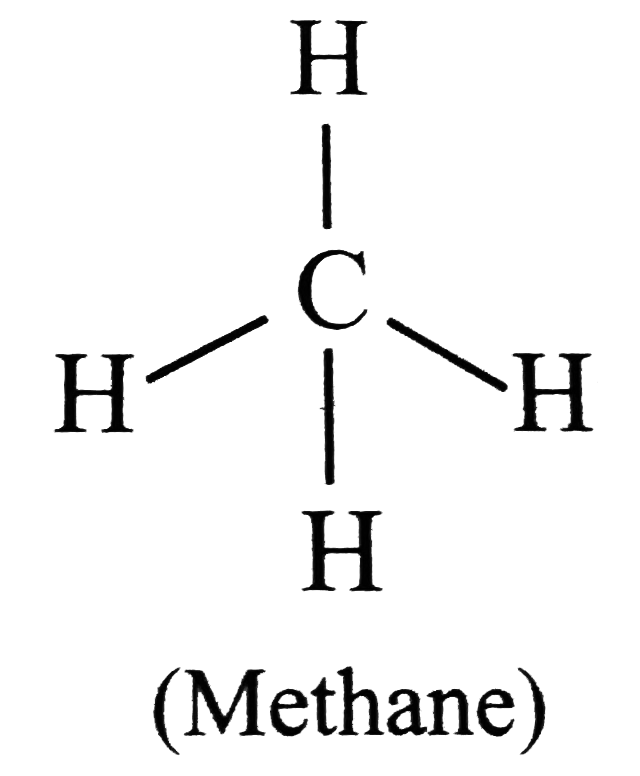
|
Similar Questions
Knowledge Check
The type of bonding in HCl molecule is
APure covalentBPolar covalentCHighly polarDH-bondingThe type of bonding in HCl molecule is
Apure covalentBpolar covalentChighly polarDH-bondingThe type of bonding in HCl molecule is
APure covalentBPolar covalentCHighly polarDHydrogen bondiing
What are fleshy fruits ? Describe the various types of fleshy fruits with suitable examples.
View SolutionHydrogen Bonding and its types
View SolutionMatch the compounds with type of hydrogen bond.

View SolutionThe type of bonding in HCI molecule is:
View SolutionThe types(s) of the bond in RNC is / are
View Solution
