(a) On bolining a minerak (A) with NaCO3 solution, a white precipirtate (B) is formed.
(b) The precipitate is fitered and the filtrate contains two conpounds (C) and (D). The componds (C) is removed by crystallisation and when CO2 is passed through the mother liquor left, (D) changes to (C).
( c) On strongly heating (C), two compounds (D) and (E) are formed.
(d) (E) on heating with cobalt oxide provides blue-coloured substance (F).
Identify (A) to (F) and give chemical reaction for (a) to (d).
(a) The mineral (A) is colemanite, Ca2B6O11.5H2O
Ca2B6O11(A).5H2O+2Na2CO3(aq)Boiled−−−→
2CaCO3⏐⏐
⏐↓+Na2B4O7(aq)(C)+2NaBO2(aq)(D)
(B), i.e white ppt. is of calcium carbonate.
(b) The filterate formed in step (a) contains borax Na2B4O7(C) and sodium metaborate, NaBO2(D).
On crystallisation, borax gets separated out in the form of white crystals. Filterate now consists of NaBO2 only. On passing CO2 though the filterate, sodium metaborate NaBO2(D) reacts to form borax Na2B4O7, i.e. (C).
NaBO2(D)+CO2→Na2B4O7(C)+Na2CO3
( c) On strongly heating (C), sodium metaborate (D) and boro tioxide (E) are formed.
Na2B4O7.10H2OΔ−−→Na2B4O7+10H2O
Na2B4O7Δ−−→2NaBO2(D)+B2O3(E)Boron trioxide
(d) On heating (E) with cobalt oxide (CoO), blue-coloured cobalt metaborate, Co(BO2)2(F) is formed.
B2O3+CoO→Co(BO2)2Cobalt metaborate(blue coloured)
Hence, (A) is Ca2B6O11.5H2O,(B) is CaCO3,(C) is Na2B4O7.10H2O,(D) is NaBO2 and (E) is Co(BO2)2.
Topper's Solved these Questions
- P-BLOCK GROUP 13 - BORON FAMILYBook:CENGAGE CHEMISTRYChapter:P-BLOCK GROUP 13 - BORON FAMILYExercise:Ex 6.1 (Subjective)
Explore 17 Videos
- P-BLOCK GROUP 13 - BORON FAMILYBook:CENGAGE CHEMISTRYChapter:P-BLOCK GROUP 13 - BORON FAMILYExercise:Ex 6.1 (Objective)
Explore 14 Videos
- P-BLOCK GROUP 13 - BORON FAMILYBook:CENGAGE CHEMISTRYChapter:P-BLOCK GROUP 13 - BORON FAMILYExercise:Exercise Archives (Subjecive)
Explore 9 Videos
- ORGANIC REACTION MECHANISMBook:CENGAGE CHEMISTRYChapter:ORGANIC REACTION MECHANISMExercise:Analytical and Descriptive
Explore 6 Videos
- P-BLOCK GROUP 14 - CARBON FAMILYBook:CENGAGE CHEMISTRYChapter:P-BLOCK GROUP 14 - CARBON FAMILYExercise:Exercises Archives (Subjective)
Explore 9 Videos
Similar Questions
Why boron does not exist as B2+ ion in solution or in compound ?
View SolutionHow does BF3 act as a catalyst in industrial process?
View SolutionState with balanced equations, what happens when aluminium is reacted with hot concentrated caustic soda solution.
View SolutionDraw the stereochemical structures of the products in the following reactions.
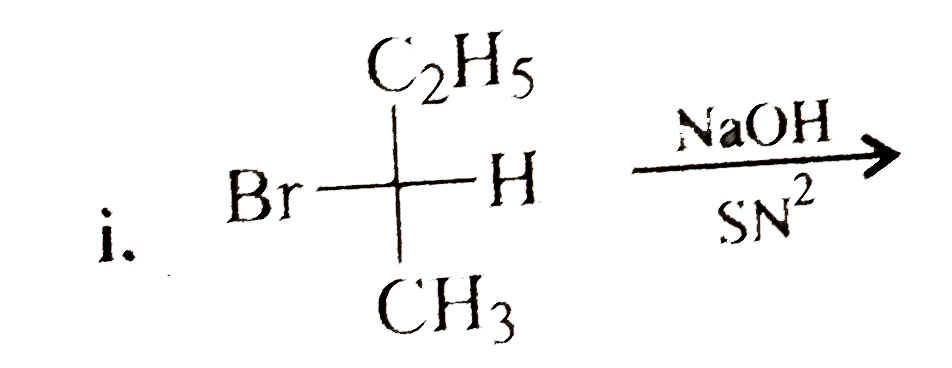 .
.
R−C≡C−RH2,Lindlar catalyst−−−−−−−−−−−→
View SolutionWrite the chemical equations involved in the extraction of lead from galena by self-reduction process.
View SolutionIf in two triangles ABC and DEF , ABDE=BCFE=CAFD , then FDECAB (b) FDEABC (c) CBAFDE (d) BCAFDE
View SolutionWhen a colourless gas (A), which is poisonous and burns with blue flame, is passed through aqueous NaOH solution, gives a compound (B). Compound (B) on heating gives (C). (C) gives a white precipitate (D) with CaCl2. Both (C) and (D) decolourises KMnO4. Identify (A) to (D).
View SolutionA compound (A) on heating in Busen flame imparts brick red colouration. (A) on heating gives CO_(2) gas and a residue (B) . The residue (B) when treated with water gives (c ) . On passing an excess of CO_(2) through (C ) in water, a clear solution (D) is obtained. On boiling (D) , compound (A) is reformed. Compound (C ) is
View SolutionA white precipitate (X) is formed when a mineral (A) is boiled with Na_(2)CO_(3) solution. The precipitate is filtered and filtrate contains two compound (Y) and (Z). The compound (Y) is removed by crystallization and when CO_(2) is passed through the filtrate obtained after crystallization, then (Z) changed to (Y) . When compound (Y) is heated, it gives two compounds (Z) and (T). Compound (T) on heating with cobalt oxide produces blue coloured substance (S) The compound (Y) in the filtrate when (A) is boiled with Na_(2)CO_(3) is
View SolutionAn inorganic compound 'A' on heating with solution of KOH , gives a gas B and the solution of compound C. gas B on ignition with excess of air gives a compound D and water. Copper sulphate gives a black precipitate on passing through its solution. White precipitate E is obtained on reaction of C with copper sulphates solution. Compound D is:
View SolutionAn inorganic compound 'A' on heating with solution of KOH, gives a gas B and the solution of compound C. gas B on ignition with excess of air gives a compound D and water. Copper sulphate gives a black precipitate on passing through its solution. White precipitate E is obtained on reaction of C with copper sulphates solution.
Compound D is:
View SolutionA salt 'A' on heating forms the oxide of the metal 'B' and a gas 'C'. 'B' is used in white washing . When C is passed through water it forms 'D'. Identify A, B, C and D. Justify the usage of 'C' for the above purpose.
View Solution(a) On bolining a mineral (A) with Na2CO3 solution, a white precipirtate (B) is formed.
(b) The precipitate is fitered and the filtrate contains two conpounds (C) and (D). The componds (C) is removed by crystallisation and when CO2 is passed through the mother liquor left, (D) changes to (C).
( c) On strongly heating (C), two compounds (D) and (E) are formed.
(d) (E) on heating with cobalt oxide provides blue-coloured substance (F).
Identify (A) to (F) and give chemical reaction for (a) to (d).
View SolutionTo a solution containing Ca2+, Ag+, Cu+ and K+ 2 M HCl is added when a white ppt. (A) is obtained. After filtration H2S is passed through the filtrate, a black ppt. (B) is formed. On removing (B) by filtration it gave white ppt. (C) with conc. Na2CO3 solution. Identify (A),(B) and (C).
View SolutionIf A={a,b},B={c,d},C={d,e}, then : {(a,c),(a,d),(a,e),(b,c),(b,d),(b,e)} is :
View Solution
