The unexpected order of acidic strength of the trihalides of boron can best be explained by.
A
pπ−pπ beack bonding
B
Hybridisation
C
Trigonal planar structure
D
None of the above
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for The unexpected order of acidic strength of the trihalides of boron can best be explained by. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
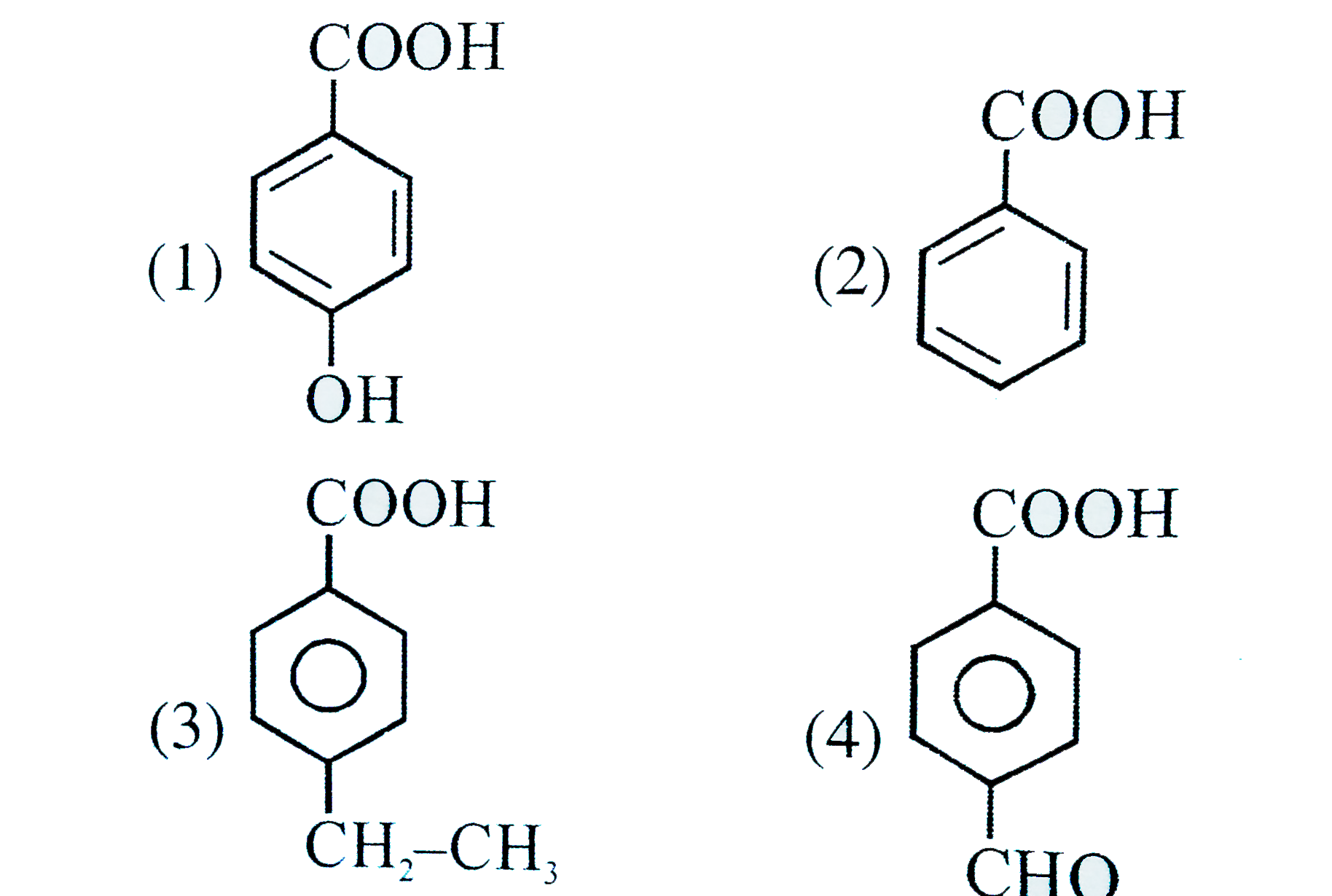
Correct order of acidic strength
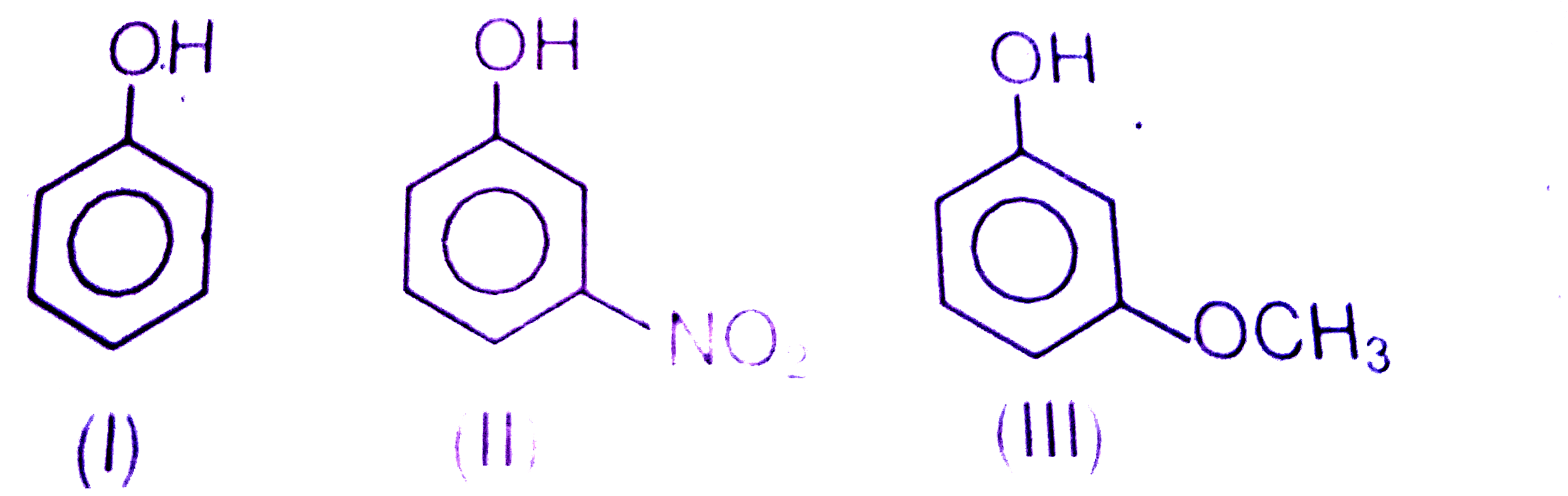 AI>II>IIIBII>III>ICI>III>IIDII>II>I
AI>II>IIIBII>III>ICI>III>IIDII>II>IThe order of acidic strenght boron trihalides is:
ABF3<BCl3<BBr3<BI3BBI3<BBr3<BCl3<BF3CBCl3<BBr3<BI3<BF3DBBr3<BCl3<BF3<BI3Correct order of acidic strengths
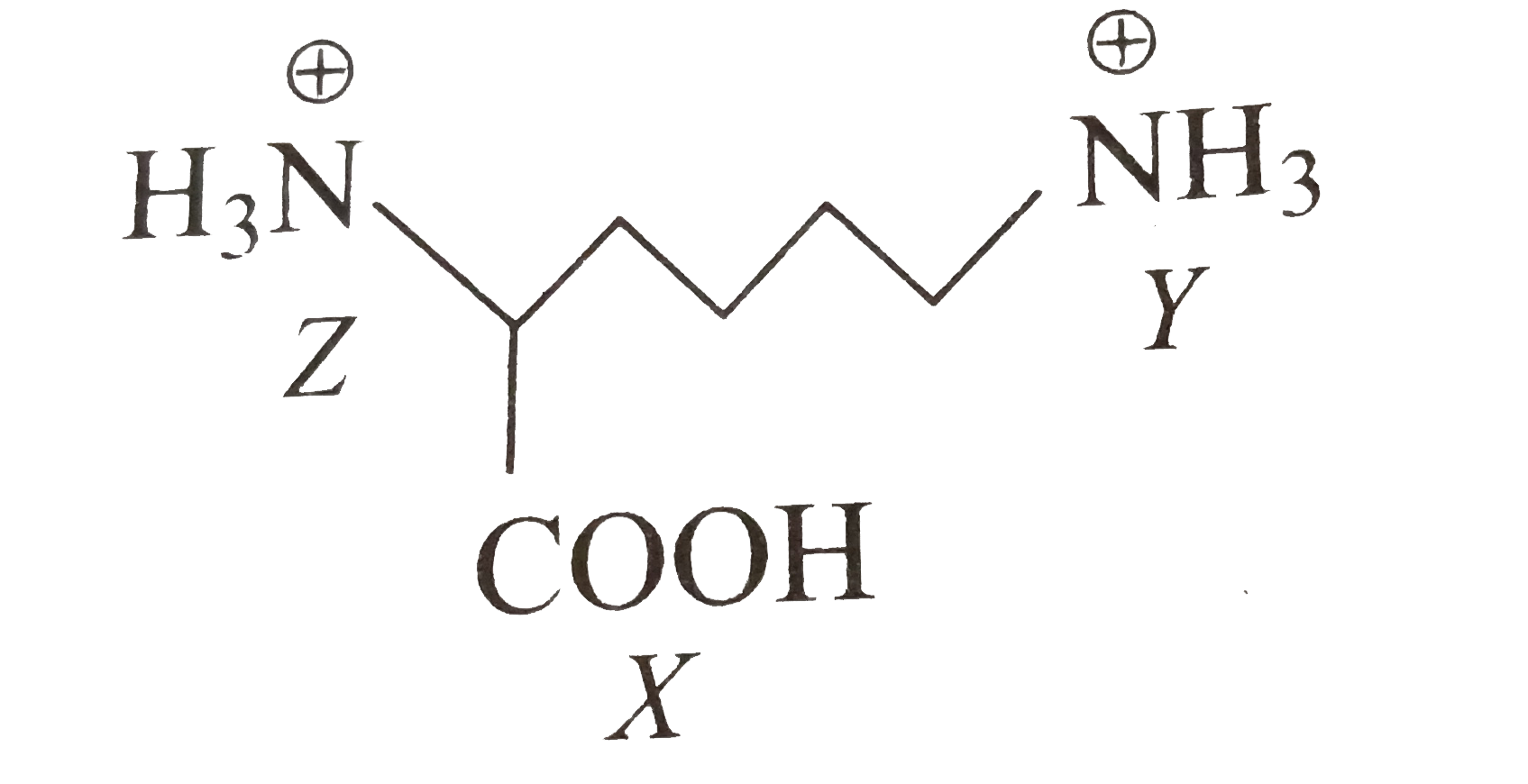 AX>Y>ZBZ>Y>XCY>Z>XDX>Z>Y
AX>Y>ZBZ>Y>XCY>Z>XDX>Z>Y