Al(OH)3 is amphoteric is nature.
Al−O and O−H bonds can be borken with equal ease in Al(OH)3.
The correct Answer is:C
Assertion (A) is true , Reason (R) is false.
The O−H bond is stronger than the Al−O bond in aluminium hydroxide.
Similar Questions
Knowledge Check
Al(OH)3 is amphoteric is nature.
Al−O and O−H bonds can be borken with equal ease in Al(OH)3.AStatement 1 is true, statement 2 is true, statement 2 si a correct explanation for statement 6BStatement 1 is true, statement 2 is true, statement 2 is not a correct explanation for statement 6CStatement 1 is true, statement 2 is falseDStatement 1 is false, statement 2 is trueAssertion (A) : Al(OH)3 is amphoteric in nature.
Reason (R) : Al−O and O−H bonds can be broken with equal ease in Al(OH)3.ABoth A and R are true and R is the correct explanation of ABBoth A and R are true but R is not a correct explanation of ACA is true but R is falseDA is false but R is trueStatement -I Al(OH)3 is amphoteric in nature.
statement -II Al-O and O-H bonds can be broken with equal case in Al(OH)3AStatement I is correct : statement II is correct : statement II is the correct explanation of statement IBStatement I is correct : statement II is correct : statement II is not the correct explanation of statement ICStatement I is correct : statement II is incorrectDStatement I is incorrect : statement II is correct
Why Al2O3 is amphoteric while B2O3 is acidic ?
View SolutionNaOH can be used to seprate Al(OH)3and Zn(OH)2 .
View SolutionAssertion: Al(OH)3 is amphoteric in nature.
Reason: Al(OH)3 is H+ donar acid as well as OH− donar base.
View SolutionStatement-1: Al(OH)3 is amphoteric in nature.
Statement-2: It cannot be used as an antacid.
View SolutionAmphoterism
Amphoteric oxides, such as aluminium oxide, are soluble both in strongly acidic and in strongly base solutions :
In acid:Al2O3(s)+6H3O+(aq)⇔2Al3+(aq)+9H2O(l)
In base:Al(OH)3(s)+OH−(aq)⇔Al(OH)−4(aq)
Dissolution of Al(OH)3 in excess base is just a special case of the effect of complex-ion formation on solubility.Al(OH)3 dissolves because excess OH− ions convert it to the soluble complex ion Al(OH)−4(aluminate ion)The effect of pH on the solubility of Al(OH)3 is shown in figure.
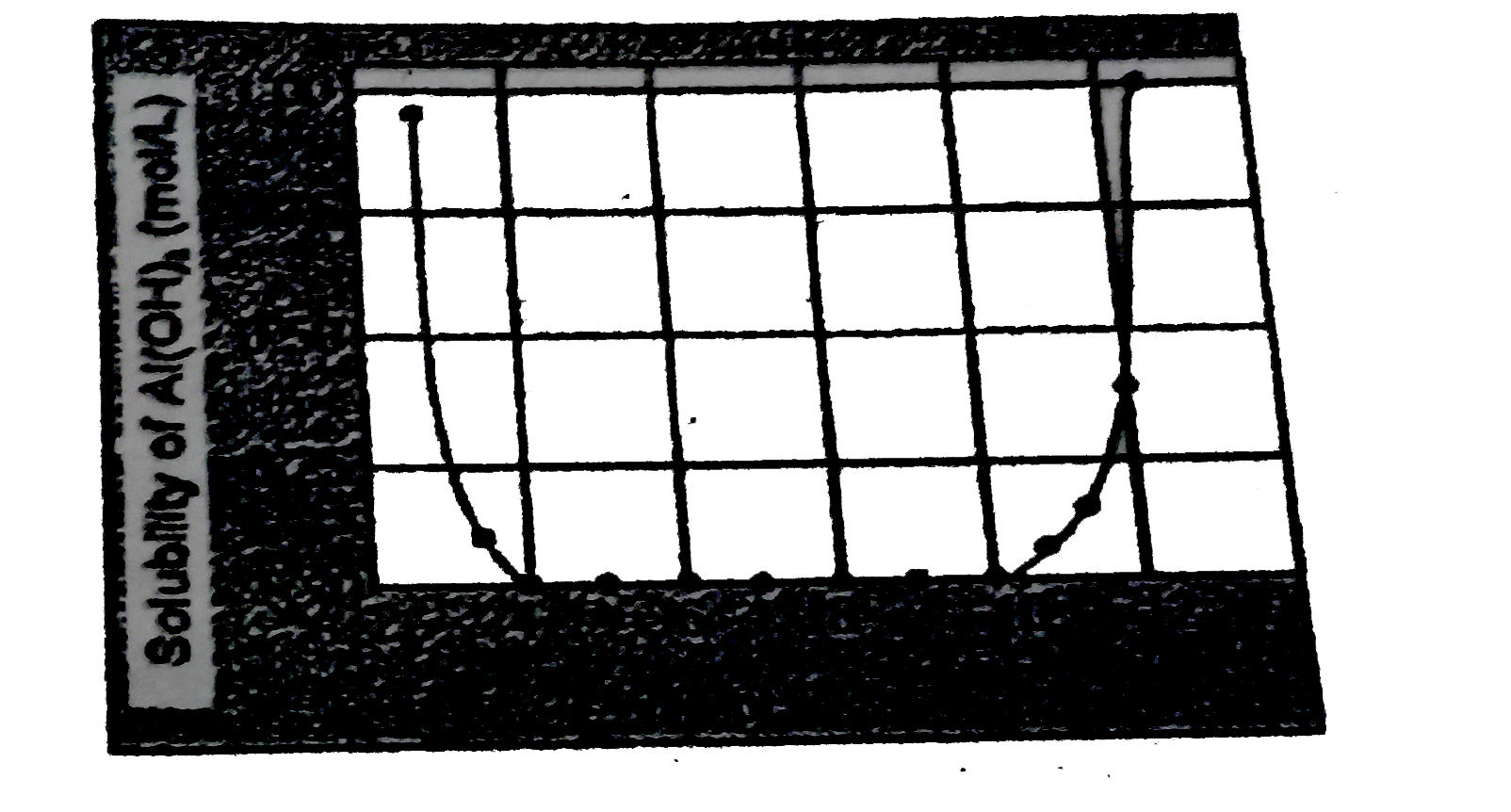
Other examples of amphoteric hydroxides include Zn(OH)2,Cr(OH)3,Sn(OH)2andPb(OH)2, which react with excess OH− ions to form the soluble complex ion Zn(OH)2−4(zincate ion),Cr(OH)−4(chromite ion),Sn(OH)−3 , Fe(OH)2andFe(OH)3 , dissolve in strong acid but not in strong base.
Zn(OH)2 is a amphoteric hydroxide and is involved in the following two equilbria in aqueous solutions
Zn(OH)2(s)⇔Zn2+(aq)+2OH−(aq),Ksp=1.2×10−17
Zn(OH)2(s)+2OH−(aq)⇔[Zn(OH)4]2−(aq),K∘=0.12
At what pH the solubility of Zn(OH)2 be minimum ?
View Solution
